Non-alcoholic fatty liver disease: A patient guideline
- PMID: 34693236
- PMCID: PMC8514420
- DOI: 10.1016/j.jhepr.2021.100322
Non-alcoholic fatty liver disease: A patient guideline
Erratum in
-
Erratum Regarding Previously Published Articles.JHEP Rep. 2024 May 18;6(6):101097. doi: 10.1016/j.jhepr.2024.101097. eCollection 2024 Jun. JHEP Rep. 2024. PMID: 38978774 Free PMC article.
Abstract
This patient guideline is intended for all patients at risk of or living with non-alcoholic fatty liver disease (NAFLD). NAFLD is the most frequent chronic liver disease worldwide and comes with a high disease burden. Yet, there is a lot of unawareness. Furthermore, many aspects of the disease are still to be unravelled, which has an important impact on the information that is given (or not) to patients. Its management requires a close interaction between patients and their many healthcare providers. It is important for patients to develop a full understanding of NAFLD in order to enable them to take an active role in their disease management. This guide summarises the current knowledge relevant to NAFLD and its management. It has been developed by patients, patient representatives, clinicians and scientists and is based on current scientific recommendations, intended to support patients in making informed decisions.
Keywords: ALD, alcohol-related or alcoholic liver disease; ASH, alcoholic steatohepatitis; BMI, body mass index; CAP, controlled attenuation parameter; CT, computed tomography; CVD, cardiovascular disease; EASD, European Association for the Study of Diabetes; EASL, European Association for the Study of the Liver; EASO, European Association for the Study of Obesity; FIB-4, fibrosis-4 index; FXR, farnesoid X receptor; GLP-1 RAs, glucagon-like receptor 1 agonists; GP, general practitioner; HCC, hepatocellular carcinoma; HDL, high-density lipoprotein; LDL, low-density lipoproteins; MRE, magnetic resonance elastography; MRI, magnetic resonance imaging; NAFL, non-alcoholic fatty liver; NAFLD, non-alcoholic fatty liver disease; NASH CRN, NASH Clinical Research Network; NASH, non-alcoholic steatohepatitis; NIT, non-invasive test; SMART, specific, measurable, achievable, relevant, timely; T1D, type 1 diabetes; T2D, type 2 diabetes.
© 2021 The Authors.
Conflict of interest statement
SMF has a senior clinical research mandate from the Fund for Scientific Research (FWO) Flanders (1802154 N) and has acted as advisor and/or lecturer for Roche, Gilead, Abbvie, Bayer, BMS, MSD, Janssen, Actelion, Astellas, Genfit, Inventiva, Intercept, Genentech, Galmed, Promethera, Coherus, NGM Bio and Julius Clinical. GM served as Advisory Board member for Sanofi, Novartis, Astra-Zeneca, Intercept and Pfizer, and participated in clinical trials by Sanofi, Eli Lilly, Gilead, GENFIT and Intercept. AK has received project grants from Novartis and Intercept. MW has nothing to disclose in relation to this publication. RD has received project grants from Novartis and Intercept. JVL received research grants from Gilead Sciences and MSD, and speaker fees from Genfit, Gilead Sciences, MSD, Intercept Pharmaceuticals and Janssen, outside of this work. SZS has nothing to disclose in relation to this publication. KH has nothing to disclose in relation to this publication. LB serves as an Advisory Board member for Novo Nordisk. GF has nothing to disclose in relation to this publication. DD served as Advisory Board member and lecture for Novo Nordisk, Sanofi, BI, Astra-Zeneca, and participated in clinical trials by, Novo Nordisk, Eli Lilly, Sanofi and BI. EW has nothing to disclose in relation to this publication. MK has nothing to disclose in relation to this publication. JW has nothing to disclose in relation to this publication. GK has nothing to disclose in relation to this publication. SV has nothing to disclose in relation to this publication. MU has nothing to disclose in relation to this publication. JM has nothing to disclose in relation to this publication. CL has nothing to disclose in relation to this publication. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures
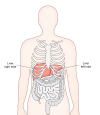



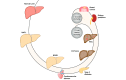







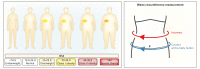



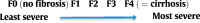











References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

