Flow-cytometry-based protocols for human blood/marrow immunophenotyping with minimal sample perturbation
- PMID: 34693361
- PMCID: PMC8517606
- DOI: 10.1016/j.xpro.2021.100883
Flow-cytometry-based protocols for human blood/marrow immunophenotyping with minimal sample perturbation
Abstract
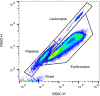
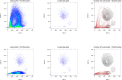
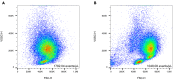
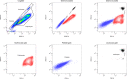
This protocol provides instructions to improve flow cytometry analysis of marrow/peripheral blood cells by avoiding erythrolytic solutions, density gradients, and washing steps. We describe two basic approaches for identifying cell surface antigens with minimal sample perturbation, which have been successfully used to identify healthy and pathologically rare cells. The greatest advantage of these approaches is that they minimize the unwanted effect caused by sample preparation, allowing for improved study of live cells at the point of analysis. For complete details on the use and execution of this protocol, please refer to Petriz et al. (2018).
Keywords: Cancer; Clinical Protocol; Flow Cytometry/Mass Cytometry; Health Sciences; Immunology; Stem Cells.
© 2021 The Author(s).
Conflict of interest statement
M.D.W. and J.A.B. are employees of Thermo Fisher Scientific, which is in the business of selling flow cytometers and flow cytometry reagents. The rest of the authors declare no competing interests.
Figures

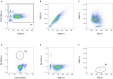

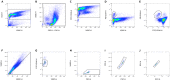
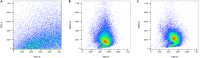
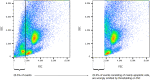
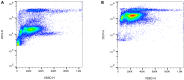
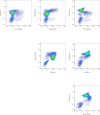

References
-
- Alvarez-Larran A., Jover Ll., Marin P., Petriz J. A multicolor, no-lyse no-wash assay for the absolute counting of CD34+ cells by flow cytometry. Cytometry. 2002;50:249–253. - PubMed
-
- Avendaño A., Sales-Pardo I., Marin L., Marin P., Petriz J. Oxidative burst assessment and neutrophil-platelet complexes in unlysed whole blood. J. Immunol. Methods. 2008;339:124–131. - PubMed
-
- Bardina J., Rico L.G., Ward M.D., Bradford J.A., Juncà J., Petriz J. Flow Cytometric Quantification of Granulocytic Alkaline Phosphatase Activity in Unlysed Whole Blood. Curr. Protoc. Cytom. 2020;93:e76. - PubMed
-
- Bohren C.F., Huffman D.R. Wiley-Interscience; 1983. Absorption and Scattering of Light by Small Particles.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

