Characteristics and outcomes of adverse events after COVID-19 vaccination
- PMID: 34693399
- PMCID: PMC8514147
- DOI: 10.1002/emp2.12565
Characteristics and outcomes of adverse events after COVID-19 vaccination
Abstract
Objectives: BNT-162b2, mRNA-1273, and Ad26.COV2.S vaccines data regarding adverse events (AEs) are scarce. In this report, we aimed to describe fatal and non-fatal possible AEs after COVID-19 vaccine administration.
Methods: An observational multicenter study investigating the causes of emergency department visits and hospital admissions within 10 days of COVID-19 vaccination. Patients who received first or second doses of COVID-19 vaccines and presented to the emergency department (ED), as well as those admitted to the hospitals or intensive care units (ICUs) were included. Causes of ED, hospital, and ICU admissions and discharges were collected based on the International Classification of Diseases, Tenth Revision (ICD-10) coding system.
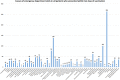
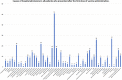
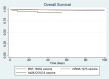
Results: Between December 2020 and March 2021, 1842 patients visited the ED within 10 days of COVID-19 vaccine administration. The mean age was 70.3 years. Overall, 1221 patients presented after the first dose of the vaccine and 653 after the second dose. Trauma (14.9%), hypertensive emergency/urgency (7.8%), generalized pain and arthralgia (5.7%), and chest pain (4.4%) were the most common causes of presentation to the ED. Of all ED presentations, mortality rate was at 2.2% (41 patients) with a median follow-up time of 68.0 days, versus 2.6% in unvaccinated ED patients. Postvaccination acute hypoxemic respiratory failure (46.3%), septic shock (24.4%), and cardiogenic shock (12.2%) were the most common causes of death.
Conclusion: Although reported AEs are not necessarily caused by the vaccination, this study provides further information about possible AEs after COVID-19 immunization, especially those requiring hospital admission. This study also supports prior data that serious AEs post vaccination are much lower than primary COVID-19 infections. Further studies are needed to investigate causalities between vaccines and reported AEs across all age groups.
Keywords: BNT‐162b2 vaccine; COVID‐19; hospital admission; mRNA vaccine; mRNA‐1273 vaccine; serious adverse events.
© 2021 The Authors. JACEP Open published by Wiley Periodicals LLC on behalf of American College of Emergency Physicians.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- COVID‐19 Vaccinations in the United States. 2021. https://covid.cdc.gov/covid‐data‐tracker/#vaccinations
-
- World Health Organization . Causality Assessment of an Adverse Event Following Immunization (AEFI). 2nd ed. World Health Organization; 2019.
-
- COVID‐19 Vaccine Reporting Systems. CDC, 2021.
LinkOut - more resources
Full Text Sources

