Dipeptidyl peptidase-4 inhibitors, glucagon-like peptide 1 receptor agonists and sodium-glucose co-transporter-2 inhibitors for people with cardiovascular disease: a network meta-analysis
- PMID: 34693515
- PMCID: PMC8812344
- DOI: 10.1002/14651858.CD013650.pub2
Dipeptidyl peptidase-4 inhibitors, glucagon-like peptide 1 receptor agonists and sodium-glucose co-transporter-2 inhibitors for people with cardiovascular disease: a network meta-analysis
Abstract
Background: Cardiovascular disease (CVD) is a leading cause of death globally. Recently, dipeptidyl peptidase-4 inhibitors (DPP4i), glucagon-like peptide-1 receptor agonists (GLP-1RA) and sodium-glucose co-transporter-2 inhibitors (SGLT2i) were approved for treating people with type 2 diabetes mellitus. Although metformin remains the first-line pharmacotherapy for people with type 2 diabetes mellitus, a body of evidence has recently emerged indicating that DPP4i, GLP-1RA and SGLT2i may exert positive effects on patients with known CVD.
Objectives: To systematically review the available evidence on the benefits and harms of DPP4i, GLP-1RA, and SGLT2i in people with established CVD, using network meta-analysis.
Search methods: We searched CENTRAL, MEDLINE, Embase, and the Conference Proceedings Citation Index on 16 July 2020. We also searched clinical trials registers on 22 August 2020. We did not restrict by language or publication status.
Selection criteria: We searched for randomised controlled trials (RCTs) investigating DPP4i, GLP-1RA, or SGLT2i that included participants with established CVD. Outcome measures of interest were CVD mortality, fatal and non-fatal myocardial infarction, fatal and non-fatal stroke, all-cause mortality, hospitalisation for heart failure (HF), and safety outcomes.
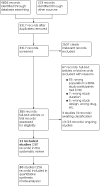
Data collection and analysis: Three review authors independently screened the results of searches to identify eligible studies and extracted study data. We used the GRADE approach to assess the certainty of the evidence. We conducted standard pairwise meta-analyses and network meta-analyses by pooling studies that we assessed to be of substantial homogeneity; subgroup and sensitivity analyses were also pursued to explore how study characteristics and potential effect modifiers could affect the robustness of our review findings. We analysed study data using the odds ratios (ORs) and log odds ratios (LORs) with their respective 95% confidence intervals (CIs) and credible intervals (Crls), where appropriate. We also performed narrative synthesis for included studies that were of substantial heterogeneity and that did not report quantitative data in a usable format, in order to discuss their individual findings and relevance to our review scope.
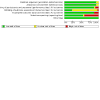
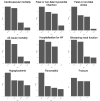
Main results: We included 31 studies (287 records), of which we pooled data from 20 studies (129,465 participants) for our meta-analysis. The majority of the included studies were at low risk of bias, using Cochrane's tool for assessing risk of bias. Among the 20 pooled studies, six investigated DPP4i, seven studied GLP-1RA, and the remaining seven trials evaluated SGLT2i. All outcome data described below were reported at the longest follow-up duration. 1. DPP4i versus placebo Our review suggests that DPP4i do not reduce any risk of efficacy outcomes: CVD mortality (OR 1.00, 95% CI 0.91 to 1.09; high-certainty evidence), myocardial infarction (OR 0.97, 95% CI 0.88 to 1.08; high-certainty evidence), stroke (OR 1.00, 95% CI 0.87 to 1.14; high-certainty evidence), and all-cause mortality (OR 1.03, 95% CI 0.96 to 1.11; high-certainty evidence). DPP4i probably do not reduce hospitalisation for HF (OR 0.99, 95% CI 0.80 to 1.23; moderate-certainty evidence). DPP4i may not increase the likelihood of worsening renal function (OR 1.08, 95% CI 0.88 to 1.33; low-certainty evidence) and probably do not increase the risk of bone fracture (OR 1.00, 95% CI 0.83 to 1.19; moderate-certainty evidence) or hypoglycaemia (OR 1.11, 95% CI 0.95 to 1.29; moderate-certainty evidence). They are likely to increase the risk of pancreatitis (OR 1.63, 95% CI 1.12 to 2.37; moderate-certainty evidence). 2. GLP-1RA versus placebo Our findings indicate that GLP-1RA reduce the risk of CV mortality (OR 0.87, 95% CI 0.79 to 0.95; high-certainty evidence), all-cause mortality (OR 0.88, 95% CI 0.82 to 0.95; high-certainty evidence), and stroke (OR 0.87, 95% CI 0.77 to 0.98; high-certainty evidence). GLP-1RA probably do not reduce the risk of myocardial infarction (OR 0.89, 95% CI 0.78 to 1.01; moderate-certainty evidence), and hospitalisation for HF (OR 0.95, 95% CI 0.85 to 1.06; high-certainty evidence). GLP-1RA may reduce the risk of worsening renal function (OR 0.61, 95% CI 0.44 to 0.84; low-certainty evidence), but may have no impact on pancreatitis (OR 0.96, 95% CI 0.68 to 1.35; low-certainty evidence). We are uncertain about the effect of GLP-1RA on hypoglycaemia and bone fractures. 3. SGLT2i versus placebo This review shows that SGLT2i probably reduce the risk of CV mortality (OR 0.82, 95% CI 0.70 to 0.95; moderate-certainty evidence), all-cause mortality (OR 0.84, 95% CI 0.74 to 0.96; moderate-certainty evidence), and reduce the risk of HF hospitalisation (OR 0.65, 95% CI 0.59 to 0.71; high-certainty evidence); they do not reduce the risk of myocardial infarction (OR 0.97, 95% CI 0.84 to 1.12; high-certainty evidence) and probably do not reduce the risk of stroke (OR 1.12, 95% CI 0.92 to 1.36; moderate-certainty evidence). In terms of treatment safety, SGLT2i probably reduce the incidence of worsening renal function (OR 0.59, 95% CI 0.43 to 0.82; moderate-certainty evidence), and probably have no effect on hypoglycaemia (OR 0.90, 95% CI 0.75 to 1.07; moderate-certainty evidence) or bone fracture (OR 1.02, 95% CI 0.88 to 1.18; high-certainty evidence), and may have no impact on pancreatitis (OR 0.85, 95% CI 0.39 to 1.86; low-certainty evidence). 4. Network meta-analysis Because we failed to identify direct comparisons between each class of the agents, findings from our network meta-analysis provided limited novel insights. Almost all findings from our network meta-analysis agree with those from the standard meta-analysis. GLP-1RA may not reduce the risk of stroke compared with placebo (OR 0.87, 95% CrI 0.75 to 1.0; moderate-certainty evidence), which showed similar odds estimates and wider 95% Crl compared with standard pairwise meta-analysis. Indirect estimates also supported comparison across all three classes. SGLT2i was ranked the best for CVD and all-cause mortality.
Authors' conclusions: Findings from both standard and network meta-analyses of moderate- to high-certainty evidence suggest that GLP-1RA and SGLT2i are likely to reduce the risk of CVD mortality and all-cause mortality in people with established CVD; high-certainty evidence demonstrates that treatment with SGLT2i reduce the risk of hospitalisation for HF, while moderate-certainty evidence likely supports the use of GLP-1RA to reduce fatal and non-fatal stroke. Future studies conducted in the non-diabetic CVD population will reveal the mechanisms behind how these agents improve clinical outcomes irrespective of their glucose-lowering effects.
Trial registration: ClinicalTrials.gov NCT03619213 NCT03087773 NCT03485222 NCT03057951 NCT04146155 NCT02917031 NCT02397421 NCT03574597 NCT03485092.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
TK declares having no conflicts of interest.
AM declares having no conflicts of interest.
YT declares having no conflicts of interest.
TS declares having no conflicts of interest.
DY declares having no conflicts of interest.
YN declares having no conflicts of interest.
WWST declares having no conflicts of interest.
JM declares having no conflicts of interest.
AR has previously received honoraria for speaking and consultancy from Boehringer Ingelheim in Poland.
YX declares having no conflicts of interest.
OW: The CRSU is a support unit funded by NIHR to provide methodological advice to NIHR‐funded evidence synthesis research. It is within the CRSU's remit to support Cochrane Reviews with research questions that are relevant to UK NHS patients. OW is the Director of the CRSU. OW also declares a research grant from Novo Nordisk (2017‐2018), received via their institution, for a study to estimate the incidence and the economic burden of cardiovascular events in type 2 diabetes mellitus in Scotland, using routine linked health data.
RP declares having no conflicts of interest.
JSWK declares having no conflicts of interest.
Figures









































Comment in
-
In CV disease, GLP-1 RAs and SGLT2 inhibitors reduce CV mortality.Ann Intern Med. 2022 Mar;175(3):JC26. doi: 10.7326/J22-0003. Epub 2022 Mar 1. Ann Intern Med. 2022. PMID: 35226528
-
DPP-4 Inhibitors, GLP-1 Receptor Agonists, and SGLT-2 Inhibitors for People With Cardiovascular Disease.Am Fam Physician. 2022 Jul;106(1):24-25. Am Fam Physician. 2022. PMID: 35839371 No abstract available.
References
References to studies included in this review
Arturi 2017 {published data only}
-
- Arturi F, Succurro E, Miceli S, Cloro C, Ruffo M, Maio R, et al. Liraglutide improves cardiac function in patients with type 2 diabetes and chronic heart failure. Endocrine 2017;57(3):464-73. - PubMed
Bhatt 2021 {published data only}
-
- Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al, SOLOIST-WHF Trial Investigators. Sotagliflozin In patients with diabetes and recent worsening heart failure. New England Journal of Medicine 2021;384(2):117-28. [PMID: ] - PubMed
-
- Effect of sotagliflozin on cardiovascular events in patients with type 2 diabetes post worsening heart failure (SOLOIST-WHF trial). Available from clinicaltrials.gov/ct2/show/NCT03521934 (first posted 11 May 2018).
Cannon 2020 {published data only}
-
- Cannon CP, McGuire DK, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, et al. Design and baseline characteristics of the eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes trial (VERTIS-CV). American Heart Journal 2018;206:11-23. - PubMed
-
- Cannon CP, McGuire DK, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, et al. Design and baseline characteristics of the evaluation of ertuglifozin efficacy and safety cardiovascular outcomes trial (VERTIS-CV). Journals of the American College of Cardiology 2018;71(11):A1825. - PubMed
-
- Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, et al, VERTIS CV Investigators. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. New England Journal of Medicine 2020;383(15):1425-35. [PMID: ] - PubMed
-
- Cardiovascular outcomes following ertugliflozin treatment in type 2 diabetes mellitus participants with vascular disease: the VERTIS CV study (MK-8835-004). Available from clinicaltrials.gov/ct2/show/NCT01986881 (first posted 19 November 2013).
Cefalu 2015 {published data only}
-
- Cefalu WT, Leiter LA, Bruin TW, Gause-Nilsson I, Sugg J, Parikh SJ. Dapagliflozin's effects on glycemia and cardiovascular risk factors in high-risk patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled study with a 28-week extension. Diabetes Care 2015;38(7):1218-27. - PMC - PubMed
-
- Efficacy and safety in patients with type 2 diabetes mellitus, cardiovascular disease and hypertension. Available from clinicaltrials.gov/ct2/show/NCT01031680 (first posted 14 December 2009).
Gantz 2017 {published data only}
-
- A study to assess cardiovascular outcomes following treatment with omarigliptin (MK-3102) in participants with type 2 diabetes mellitus (MK-3102-018). Available from clinicaltrials.gov/ct2/show/NCT01703208 (first posted 12 December 2017).
Green 2015 {published data only}
-
- Bethel MA, Engel SS, Ding J, Josse RG, Holman RR. Impact of sitagliptin on cardiovascular and safety-related outcomes in insulin-treated type 2 diabetes: the TECOS experience. Diabetologia 2016;59(1):S369.
-
- Bethel MA, Engel SS, Garg J, Stevens SR, Lokhnygina Y, Josse RG. Time to insulin in the trial evaluating cardiovascular outcomes with sitagliptin (TECOS). Diabetes 2017;66:A316. - PubMed
-
- Bethel MA, Green JB, Milton J, Tajar A, Engel SS, Califf RM, et al, TECOS Executive Committee. Regional, age and sex differences in baseline characteristics of patients enrolled in the Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS). Diabetes, Obesity and Metabolism 2015;17(4):395-402. - PubMed
-
- Bhatt AS, Luo N, Solomon N, Pagidipati NJ, Ambrosio G, Green JB, et al, TECOS Study Group. International variation in characteristics and clinical outcomes of patients with type 2 diabetes and heart failure: Insights from TECOS. American Heart Journal 2019;218:57-65. - PubMed
Hernandez 2018 {published data only}
-
- A long term clinical study to determine the effect of albiglutide, when added to standard blood glucose lowering therapies, on major cardiovascular events in patients with Type 2 diabetes. http://www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2014-001824-32-CZ (date of regisration 13 January 2015).
-
- Effect of albiglutide, when added to standard blood glucose lowering therapies, on major cardiovascular events in subjects with type 2 diabetes mellitus. Available from clinicaltrials.gov/ct2/show/NCT02465515 (first posted 8 June 2015).
-
- Green JB, Hernandez AF, D'Agostino RB, Granger CB, Janmohamed S, Jones NP, et al. Harmony outcomes: a randomized, double-blind, placebo-controlled trial of the effect of albiglutide on major cardiovascular events in patients with type 2 diabetes mellitus: rationale, design, and baseline characteristics. American Heart Journal 2018;203:30-8. - PubMed
-
- Hernandez AF, Green JB, Janmohamed S, D'Agostino RB Sr, Granger CB, Jones NP, et al, Harmony Outcomes committees and investigators. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 2018;392(10157):1519-29. - PubMed
Holman 2017 {published data only}
-
- Badjatiya A, Merrill P, Buse JB, Goodman SG, Katona B, Iqbal N, et al. Clinical outcomes in patients with type 2 diabetes mellitus and peripheral artery disease: results from the EXSCEL trial. Circulation: Cardiovascular Interventions 2019;12(12):e008018. - PubMed
-
- Badjatiya A, Merrill P, Buse JB, Goodman SG, Katona B, Iqbal N, et al. Patients with type 2 diabetes mellitus and peripheral artery disease: results from the EXSCEL trial. Journal of the American College of Cardiology 2019;73(9):2040. - PubMed
-
- Bethel MA, Mentz RJ, Merrill P, Buse JB, Chan JC, Goodman SG, et al. Renal outcomes in the exenatide study of cardiovascular event lowering (EXSCEL). Diabetes 2018;67:A138.
-
- Bethel MA, Stevens SR, Buse JB, Choi J, Gustavson SM, Iqbal N, et al. Exploring the possible impact of unbalanced open-label drop-In of glucose-lowering medications on EXSCEL outcomes. Circulation 2020;141(17):1360-70. - PubMed
Husain 2019 {published data only}
-
- A trial investigating the cardiovascular safety of oral semaglutide in subjects with type 2 diabetes. Available from www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2015-003563-10-ES (date of registration 19 April 2016).
-
- A trial investigating the cardiovascular safety of oral semaglutide in subjects with type 2 diabetes (PIONEER 6). Available from clinicaltrials.gov/ct2/show/NCT02692716 (first posted 26 February 2016).
-
- Bain SC, Mosenzon O, Arechavaleta R, Bogdański P, Comlekci A, Consoli A, et al. Cardiovascular safety of oral semaglutide in patients with type 2 diabetes: Rationale, design and patient baseline characteristics for the PIONEER 6 trial. Diabetes, Obesity and Metabolism 2019;21(3):499-508. - PMC - PubMed
-
- Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al, PIONEER 6 Investigators. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. New England Journal of Medicine 2019;381(9):841-51. - PubMed
Jorsal 2017 {published data only}
-
- Johansen ML, Rasmussen J, Holmager P, Gustafsson I, Schou M, Faber J, et al. Treatment with liraglutide increases 24-hour heart rate in chronic heart failure patients with and without type 2 diabetes: a sub-study from the LIVE randomised clinical trial. Diabetologia 2016;59(1):S368.
-
- Jorsal A, Kistorp C, Holmager P, Tougaard RS, Nielsen R, Hänselmann A, et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double-blind, randomised, placebo-controlled trial. European Journal of Heart Failure 2017;19(1):69-77. - PubMed
-
- Jorsal A, Wiggers H, Holmager P, Nilsson B, Nielsen R, Boesgaard TW, et al. A protocol for a randomised, double-blind, placebo-controlled study of the effect of LIraglutide on left VEntricular function in chronic heart failure patients with and without type 2 diabetes (The LIVE Study). BMJ Open 2014;4(5):e004885. - PMC - PubMed
-
- Kistorp C, Holmager P, Rasmussen J, Schou M, Faber J, Tarnow L, et al. The effect of liraglutide on body composition among patients with heart failure with and without type 2 diabetes: a sub-study from the LIVE randomised clinical trial. Diabetologia 2016;59(1):S367.
-
- Liraglutide in chronic heart failure. A randomised, double-blind, placebo-controlled study of the effect of LIraglutide on the left VEntricular function in patients with chronic heart failure with and without type 2 diabetes (The LIVE-study). www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2011-002468-26-DK (date of registration 12 August 2011).
Kato 2017 {published data only}
-
- Assessment in patients with Type 2 diabetes mellitus in addition to cOronary artery disease after Percutaneous coronary intervention with regard to Sitagliptin-induced COronary plaque REgression (TOP-SCORE). Available from upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=bro....
-
- Kato Y, Iwata A, Zhang B, Miura SI, Imaizumi S, Kuwano T, et al. Effects of dipeptidyl peptidase-4 inhibitor sitagliptin on coronary atherosclerosis as assessed by intravascular ultrasound in type 2 diabetes mellitus with coronary artery disease. IJC Metabolic and Endocrine 2017;16:1-9.
Leiter 2014 {published data only}
-
- Efficacy and safety in patients with type 2 diabetes mellitus and cardiovascular disease. Available from clinicaltrials.gov/ct2/show/NCT01042977 (first posted 6 January 2010).
-
- Leiter LA, Cefalu WT, Bruin TW, Gause-Nilsson I, Sugg J, Parikh SJ. Dapagliflozin added to usual care in individuals with type 2 diabetes mellitus with preexisting cardiovascular disease: a 24-week, multicenter, randomized, double-blind, placebo-controlled study with a 28-week extension. Journal of the American Geriatrics Society 2014;62(7):1252-62. - PubMed
Margulies 2016 {published data only}
-
- Functional impact of GLP-1 for heart failure treatment (FIGHT). Available from clinicaltrials.gov/ct2/show/NCT01800968 (first posted 28 February 2013).
-
- Margulies KB, Anstrom KJ, Redfield MM, Givertz MM, Cole R, Mann DL, et al, NHLBI Heart Failure Clinical Research Network. A randomized trial of liraglutide for high-risk heart failure patients with reduced ejection fraction. Circulation 2015;132(23):2268.
Marso 2016a {published data only}
-
- Asirvatham AJ, Verma S, Bain S, Bhatt DL, Leiter LA, Mazer CD, et al. The glucagon-like peptide-1 receptor agonists liraglutide and semaglutide improve cardiovascular and renal outcomes across most body mass index categories in type 2 diabetes: results of the LEADER and SUSTAIN 6 trials. Indian Journal of Endocrinology and Metabolism 2019;23(7):S16.
-
- Athyros VG, Katsiki N, Tentolouris N. Editorial: Do some glucagon-like-peptide-1 receptor agonists (GLP-1 RA) reduce macrovascular complications of type 2 diabetes mellitus? A commentary on the liraglutide effect and action in diabetes: evaluation of cardiovascular outcome results (LEADER) trial. Current Vascular Pharmacology 2016;14(5):469-73. - PubMed
-
- Bain S, Zinman B, Marso SP, Christiansen E, Calanna S, Rasmussen S, et al. Severe hypoglycaemia, cardiovascular outcomes and death: experience from the 'Liraglutide Effect and Action in Diabetes: evaluation of Cardiovascular Outcome Results' (LEADER) trial. Diabetic Medicine 2018;35:11.
-
- Bhansali A, Nauck MA, Buse JB, Monk-Hansen T, Rasmussen S, Treppendahl MB, et al. Detailed description of myocardial infarctions and the various subtypes in the LEADER trial. Indian Journal of Endocrinology and Metabolism 2017;21(8):S25.
-
- Consoli A, Bergenstal R, Daniels G, Mann J, Nissen S, Pocock S, et al. Liraglutide effect and action in diabetes: evaluation of cardiovascular outcome results (leader) trial: rationale and study design. High Blood Pressure & Cardiovascular Prevention 2012;19(2):104.
Marso 2016b {published data only}
-
- A long-term, randomised, double-blind, placebo-controlled, multinational, multi-centre trial to evaluate cardiovascular and other long-term outcomes with semaglutide in subjects with type 2 diabetes. Available from www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2012-002839-28-PL (date of registration 15 January 2013).
-
- Bain S, ReaR, Warren M, Holst AG, Vrazic H, Madsbad S. Semaglutide consistently reduces cardiovascular risk in patients with type 2 diabetes regardless of baseline cardiovascular risk level: post hoc analyses of the SUSTAIN trial programme. European Heart Journal 2018;39(suppl_1):ehy565.P2859.
-
- Consoli A, Bain SC, Davies M, Lingvay I, Bergan EQ, Hansen O, et al. Semaglutide provides sustained reductions in body weight over 2 years in subjects with type 2 diabetes (SUSTAIN 6). Diabetologia 2017;60(1):S4.
-
- Leiter LA, Bain SC, Hramiak I, Jódar E, Madsbad S, Gondolf T, et al. Cardiovascular risk reduction with once-weekly semaglutide in subjects with type 2 diabetes: a post hoc analysis of gender, age, and baseline CV risk profile in the SUSTAIN 6 trial. Cardiovascular Diabetology 2019;18(1):73. - PMC - PubMed
-
- Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. New England Journal of Medicine 2016;375(19):1834-44. - PubMed
McMurray 2018 {published data only}
-
- Effect of vildagliptin on left ventricular function in patients with type 2 diabetes and congestive heart failure. Available from clinicaltrials.gov/ct2/show/NCT00894868 (first posted 7 May 2009).
-
- McMurray JJ, Ponikowski P, Bolli GB, Lukashevich V, Kozlovski P, Kothny W, et al. Effects of vildagliptin on ventricular function in patients with type 2 diabetes mellitus and heart failure: a randomized placebo-controlled trial. JACC: Heart Failure 2018;6(1):8-17. - PubMed
McMurray 2019 {published data only}
-
- DAPA-HF. Available from www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2018-002614-12-ES (date of registration 18 January 2019).
-
- Dewan P, Solomon SD, Jhund PS, Inzucchi SE, Køber L, Kosiborod MN, et al. Efficacy and safety of sodium-glucose co-transporter 2 inhibition according to left ventricular ejection fraction in DAPA-HF. European Journal of Heart Failure 2020;22(7):1247-58. - PubMed
-
- Docherty K, Jackson A, Inzucchi SE, Jhund P, Kober L, Kosiborod M, et al. Consistent benefit of dapagliflozin according to background therapy patients with HFrEF: an analysis of the DAPA-HF trial. Journal of the American College of Cardiology 2020;75(11):656.
-
- Jhund P, Adamson C, Inzucchi SE, Kosiborod M, Langkilde AM, Martinez F, et al. Effect of treatment with dapagliflozin is consistent across the range of body mass index in patients with HFrEF: an analysis of the DAPA-HF trial. Journal of the American College of Cardiology 2020;75(11):673.
Neal 2017a {published data only}
-
- Arnott C, Neuen BL, Heerspink HJL, Figtree GA, Kosiborod M, Lam CS, et al. The effects of combination canagliflozin and glucagon-like peptide-1 receptor agonist therapy on intermediate markers of cardiovascular risk in the CANVAS program. International Journal of Cardiology 2020;318:126-9. - PubMed
-
- CANVAS - CANagliflozin cardioVascular Assessment Study (CANVAS). clinicaltrials.gov/ct2/show/NCT01032629 (first posted 15 December 2009).
-
- Figtree GA, Rådholm K, Barrett TD, Perkovic V, Mahaffey KW, Zeeuw D, et al. Effects of canagliflozin on feart failure outcomes associated with preserved and reduced ejection fraction in type 2 diabetes mellitus. Circulation 2019;139(22):2591-3. - PubMed
-
- Figtree GA, Rådholm K, Barrett TD, Perkovic V, Mahaffey KW, Zeeuw D, et al. Effects of canagliflozin on heart failure outcomes with and without preserved ejection fraction in type 2 diabetees: results from the CANVAS program. Journal of the American College of Cardiology 2019;73(9_Supplement_1):685.
-
- Fulcher G, Davies M, Tsoukas M, Desai M, Wysham C. Effects of canagliflozin on HbA1c and changes in antihyperglycaemic agents in the CANVAS programme. Diabetologia 2018;61:S320-□1.
Neal 2017b {published data only}
-
- A study of the effects of canagliflozin (JNJ-28431754) on renal endpoints in adult participants with type 2 diabetes mellitus. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2013-003050-25-IT (date of registration 25 November 2013).
-
- A study of the effects of canagliflozin (JNJ-28431754) on renal endpoints in adult participants with type 2 diabetes mellitus (CANVAS-R). clinicaltrials.gov/ct2/show/NCT01989754 (first posted 21 November 2013).
-
- Zeeuw D, Neal B, Perkovic V, Mahaffey KW, Fulcher G, Desai M, et al. Rationale, design, and analysis of the canagliflozin cardiovascular assessment study-renal (canvas-r)-a randomized, placebo-controlled trial. Circulation 2016;134:A18957.
-
- Neal B, Perkovic V, Mahaffey KW, Zeeuw D, Fulcher G, Erondu N, et al, CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. New England Journal of Medicine 2017;377(7):644-57. [PMID: ] - PubMed
Packer 2020 {published data only}
-
- Empagliflozin outcome trial in patients with chronic heart failure EMPEROR-Reduced. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2016-002280-34-BE (date of registration 21 March 2018).
-
- Empagliflozin outcome trial in patients with chronic heart failure with reduced ejection fraction (EMPEROR-Reduced). clinicaltrials.gov/ct2/show/NCT03057977 (first posted 20 February 2017).
-
- Faiez Zannad F, Filippatos G, Butler J, Salsali A, Kimura K, Schnee J, et al. Design and rationale of the EMPagliflozin outcome trial in patients with chronic heart failure (EMPEROR-Reduced). European Journal of Heart Failure 2018;20:441.
-
- Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al, EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. New England Journal of Medicine 2020;383(15):1413-24. [PMID: ] - PubMed
-
- Packer M, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, et al. Evaluation of the effect of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality of patients with chronic heart failure and a reduced ejection fraction: rationale for and design of the EMPEROR-Reduced trial. European Journal of Heart Failure 2019;21(10):1270-8. - PubMed
Pfeffer 2015 {published data only}
-
- Bentley-Lewis R, Aguilar D, Riddle MC, Claggett B, Diaz R, Dickstein K, et al. Rationale, design, and baseline characteristics in Evaluation of LIXisenatide in Acute Coronary Syndrome, a long-term cardiovascular end point trial of lixisenatide versus placebo. American Heart Journal 2015;169(5):631-8. - PubMed
-
- Evaluation of cardiovascular outcomes in patients with type 2 diabetes after acute coronary syndrome during treatment with AVE0010 (lixisenatide). www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2009-012852-26-BG (date of registration 26 November 2010).
-
- Evaluation of cardiovascular outcomes in patients with type 2 diabetes after acute coronary syndrome during treatment with AVE0010 (Lixisenatide) (ELIXA). clinicaltrials.gov/ct2/show/NCT01147250 (first posted 22 June 2010).
-
- Muskiet MH, Tonneijck L, Huang Y, Liu M, Saremi A, Heerspink HJL, et al. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo-controlled trial. Lancet Diabetes & Endocrinology 2018;6(11):P859-69. - PubMed
-
- Muskiet MHA, Tonneijck L, Huang Y, Liu M, Saremi A, Heerspink HJL, et al. Lixisenatide and renal outcomes in patients with type 2 diabetes-a post-hoc analysis of the elixa trial. Diabetes 2018;67:A280. - PubMed
Phrommintikul 2019 {published data only}
-
- Effects of DPP4 inhibitor versus SGLT2 inhibitor. clinicaltrials.gov/ct2/show/NCT03178591 (first posted 7 June 2017).
-
- Phrommintikul A, Wongcharoen W, Kumfu S, Jaiwongkam T, Gunaparn S, Chattipakorn S, et al. Effects of dapagliflozin vs vildagliptin on cardiometabolic parameters in diabetic patients with coronary artery disease: a randomised study. British Journal of Clinical Pharmacology 2019;85(6):1337-47. - PMC - PubMed
Rosenstock 2019 {published data only}
-
- Cardiovascular and renal microvascular outcome study with linagliptin in patients with type 2 diabetes mellitus (CARMELINA). clinicaltrials.gov/ct2/show/NCT01897532 (first posted 12 July 2013).
-
- Cooper ME, Rosenstock J, Kadowaki T, Seino Y, Wanner C, Schnaidt S, et al. Cardiovascular and kidney outcomes of linagliptin treatment in older people with type 2 diabetes and established cardiovascular disease and/or kidney disease: A prespecified subgroup analysis of the randomized, placebo-controlled CARMELINA R trial. Diabetes, Obesity & Metabolism 2020;22(7):1062-73. - PMC - PubMed
-
- Inagaki N, Yang W, Watada H, Ji L, Schnaidt S, Pfarr E, et al. Linagliptin and cardiorenal outcomes in Asians with type 2 diabetes mellitus and established cardiovascular and/or kidney disease: subgroup analysis of the randomized CARMELINA R trial. Diabetology International 2020;11(2):129-41. - PMC - PubMed
-
- Marx N, McGuire DK, Johansen O, Rosenstock J, Kahn SE, Cooper ME, et al. First plus recurrent CV and hospitalization events in the CArdiovascular and Renal Microvascular outcomE study with LINAgliptin (CARMELINA) in patients with type 2 diabetes and cardiorenal disease. European Heart Journal 2019;40:3877.
-
- Marx N, McGuire DK, Johansen OE, Rosenstock J, Kahn SE, Cooper ME, et al. Analyses of first plus recurrent cardiovascular and hospitalization events in the cardiovascular and renal microvascular outcome study with linagliptin (CARMELINA) in patients with type 2 diabetes and cardiorenal disease. Diabetes 2019;68(Supplement 1):-.
Scirica 2013 {published data only}
-
- Bergmark BA, Bhatt DL, McGuire DK, Cahn A, Mosenzon O, Steg PG, et al. Metformin use and clinical outcomes among patients with diabetes mellitus with or without heart failure or kidney dysfunction: observations from the SAVOR-TIMI 53 trial. Circulation 2019;140(12):1004-14. - PubMed
-
- Cahn A, Mosenzon O, Bhatt DL, Leibowitz G, Yanuv I, Rozenberg A, et al. Hypoglycaemia manifestations and recurrent events: lessons from the SAVOR-TIMI 53 outcome study. Diabetes, Obesity & Metabolism 2017;19(7):1045-50. - PubMed
-
- Cavender MA, Scirica BM, Braunwald E, Raz I, Mosenzon O, Im K, et al. Major hypoglycemia is associated with cardiovascular death and hospitalization for heart failure: findings from SAVOR-TIMI 53. European Heart Journal 2014;35:342.
-
- Cavender MA, Scirica BM, Raz I, Steg PG, McGuire DK, Leiter LA, et al. Cardiovascular outcomes of patients in SAVOR-TIMI 53 by baseline hemoglobin A1c. American Journal of Medicine 2016;129(3):340e1-8. - PubMed
-
- Does saxagliptin reduce the risk of cardiovascular events when used alone or added to other diabetes medications (SAVOR- TIMI 53). clinicaltrials.gov/ct2/show/NCT01107886 (first posted 21 April 2010).
Shimizu 2020 {published data only}
-
- Effect of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: multi-center placebo-controlled double-blind randomized trial. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000034442 (first date of study disclosure 1 December 2017).
-
- Effect of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: multi-center placebo-controlled double-blind randomized trial. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000030158 (date of registration 1 December 2017).
Tanaka 2019 {published data only}
-
- Effect of empagliflozin on endothelial function in cardiovascular high risk diabetes mellitus: multi-center placebo-controlled double-blind randomized trial. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000028197 (date of disclosure 21 October 2016).
-
- Tanaka A, Shimabukuro M, Machii N, Teragawa H, Okada Y, Shima KR, et al. Effect of empagliflozin on endothelial function in patients with type 2 diabetes and cardiovascular disease: results from the multicenter, randomized, placebo-controlled, double-blind EMBLEM trial. Diabetes Care 2019;42(10):e159-61. - PubMed
-
- Tanaka A, Shimabukuro M, Machii N, Teragawa H, Okada Y, Shima KR, et al. Secondary analyses to assess the profound effects of empagliflozin on endothelial function in patients with type 2 diabetes and established cardiovascular diseases: The placebo-controlled double-blind randomized effect of empagliflozin on endothelial function in cardiovascular high risk diabetes mellitus: multi-center placebo-controlled double-blind randomized trial. Journal of Diabetes Investigation 2020;11(6):1551-63. - PMC - PubMed
-
- Tanaka A, Shimabukuro M, Machii N, Teragawa H, Okada Y, Shima KR, et al. Short-term effect of empagliflozin on endothelial function in patients with type 2 diabetes and cardiovascular diseases: a placebo-controlled randomised trial (EMBLEM). Diabetologia 2019;62:S560-61.
Tanaka 2020 {published data only}
-
- Safety of canagliflozin in diabetic patients with chronic heart failure: randomized, non-inferiority trial. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000020483 (date of disclosure 25 May 2015).
-
- Safety of canagliflozin in diabetic patients with chronic heart failure: randomized, non-inferiority trial. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000017669 (date of registration 25 May 2015).
Verma 2019 {published data only}
-
- Bami K, Gandhi S, Leong-Poi H, Yan AT, Ho E, Zahrani M, et al. Effects of empagliflozin on left ventricular remodeling in patients with type 2 diabetes and coronary artery disease: echocardiographic substudy of the EMPA-HEART CardioLink-6 randomized clinical trial. Journal of the American Society of Echocardiography 2020;33(5):644-6. - PubMed
-
- Effects of Empagliflozin on cardiac structure in patients with type 2 diabetes (EMPA-HEART). clinicaltrials.gov/ct2/show/NCT02998970 (first posted 21 December 2016).
-
- Mason T, Verma S, Yan AT, Chowdhury B, Zuo F, Thorpe KE, et al. The effect of empagliflozin on myocardial extracellular matrix expansion in patients with type 2 diabetes and coronary artery disease. Circulation 2019;140:A13456.
-
- Opingari E, Verma S, Mazer CD, Connelly KA, Yan AT, Zuo F, et al. Tubular protection with empagliflozin treatment in subjects with type 2 diabetes and cardiovascular disease: a sub-analysis of the EMPA-HEART CardioLink-6 trial. Diabetologia 2019;62:S330-1.
Wang 2020 {published data only}
-
- Wang Q, Wang D, Cheng A, Sun FY, Li Z. Comparison between the effects of sitagliptin and liraglutide on blood glucose and cognitive function of patients with both type 2 diabetes mellitus and post-stroke mild cognitive impairment. International Journal of Clinical and Experimental Medicine 2020;13(2):1219-27.
White 2013 {published data only}
-
- Bohula EA, Cannon CP, White WB, Jarolim P, Bergmark BA, Kupfer S, et al. Multiple biomarker approach predicts increasing risk of recurrent CV events in patients with diabetes mellitus in the examine randomized trial. Circulation 2016;134:A19408.
-
- Cardiovascular outcomes study of alogliptin in patients with type 2 diabetes and acute coronary syndrome (EXAMINE). clinicaltrials.gov/ct2/show/NCT00968708 (first posted 31 August 2009).
Zinman 2015 {published data only}
-
- Böhm M, Fitchett D, Ofstad AP, Brueckmann M, Kaspers S, George JT, et al. Heart failure and renal outcomes according to baseline and achieved blood pressure in patients with type 2 diabetes: results from EMPA-REG OUTCOME. Journal of Hypertension 2020;38(9):1829-40. - PubMed
-
- Böhm M, Slawik J, Brueckmann M, Mattheus M, George JT, Ofstad AP, et al. Efficacy of empagliflozin on heart failure and renal outcomes in patients with atrial fibrillation: data from the EMPA-REG OUTCOME trial. European Journal of Heart Failure 2020;22(1):126-35. - PubMed
-
- BI 10773 (empagliflozin) cardiovascular outcome event trial in type 2 diabetes mellitus patients (EMPA-REG OUTCOME). clinicaltrials.gov/ct2/show/NCT01131676 (first posted 27 May 2010).
-
- Butler J, Savarese G, Sattar N, Januzzi J, Verma S, Lund LH, et al. Empagliflozin is associated with a lower risk of post-acute heart failure rehospitalization and mortality: insights from the EMPA-REG OUTCOME trial. Journal of the American College of Cardiology 2019;73(9_Supplement_1):663.
-
- Butler J, Zannad F, Fitchett D, Zinman B, Koitka-Weber A, Eynatten M, et al. Empagliflozin and kidney outcomes in patients with or without heart failure at baseline: insights from the EMPA-REG OUTCOME trial. Diabetologia 2018;61:S323.
References to studies excluded from this review
Anholm 2014 {published data only}
-
- Anholm C, Kumarathurai P, Klit MS, Kristiansen OP, Nielsen OW, Ladelund S, et al. Adding liraglutide to the backbone therapy of biguanide in patients with coronary artery disease and newly diagnosed type-2 diabetes (the AddHope2 study): a randomised controlled study protocol. BMJ Open 2014;4(7):e005942. - PMC - PubMed
Arjona 2013 {published data only}
Arnott 2020 {published data only}
-
- Arnott C, Li JW, Cannon CP, Neuen BL, Heerspink HJL, Neal B, et al. The effects of canagliflozin on heart failure and cardiovascular death by baseline participant characteristics: analysis of the CREDENCE trial. Journal of the American College of Cardiology 2020;75(11 Supplement 1):674.
Bajaj 2020 {published data only}
-
- Bajaj HS, Raz I, Mosenzon O, Murphy SA, Rozenberg A, Yanuv I, et al. Cardiovascular and renal benefits of dapagliflozin in patients with short and long-standing type 2 diabetes: Analysis from the DECLARE-TIMI 58 trial. Diabetes, Obesity and Metabolism 2020;22(7):1122-31. - PubMed
Bays 2017 {published data only}
-
- Bays HE, Sartipy P, Xu J, Sjöström CD, Underberg JA. Dapagliflozin in patients with type II diabetes mellitus, with and without elevated triglyceride and reduced high-density lipoprotein cholesterol levels. Journal of Clinical Lipidology 2017;11(2):450-8.e1. - PubMed
Besch 2017 {published data only}
-
- Besch G, Perrotti A, Mauny F, Puyraveau M, Baltres M, Flicoteaux G, et al. Clinical effectiveness of intravenous exenatide infusion in perioperative glycemic control after coronary artery bypass graft surgery: a phase II/III randomized trial. Anesthesiology 2017;127(5):775-87. - PubMed
Bremholm 2017 {published data only}
Brenner 2016 {published data only}
-
- Brenner C, Adrion C, Grabmaier U, Theisen D, Ziegler F, Leber A, et al. Sitagliptin plus granulocyte colony-stimulating factor in patients suffering from acute myocardial infarction: A double-blind, randomized placebo-controlled trial of efficacy and safety (SITAGRAMI trial). International Journal of Cardiology 2016;205:23-30. - PubMed
Brown 2017 {published data only}
Brown 2020 {published data only}
Brown‐Frandsen 2019 {published data only}
-
- Brown-Frandsen K, Emerson SS, McGuire DK, Pieber TR, Poulter NR, Pratley RE, et al, DEVOTE Study Group. Lower rates of cardiovascular events and mortality associated with liraglutide use in patients treated with basal insulin: A DEVOTE subanalysis (DEVOTE 10). Diabetes, Obesity and Metabolism 2019;21(6):1437-44. - PMC - PubMed
Cahn 2020a {published data only}
-
- Cahn A, Raz I, Bonaca M, Mosenzon O, Murphy SA, Yanuv I, et al. Safety of dapagliflozin in a broad population of patients with type 2 diabetes: Analyses from the DECLARE-TIMI 58 study. Diabetes, Obesity and Metabolism 2020;22(8):1357-68. - PubMed
Cahn 2020b {published data only}
-
- Cahn A, Mosenzon O, Wiviott SD, Rozenberg A, Yanuv I, Goodrich EL, et al. Safety and efficacy of dapagliflozin in the elderly: analysis from the DECLARE TIMI 58 study. Diabetes Care 2020;43(2):468-75. - PubMed
Cannon 2020b {published data only}
-
- Cannon CP, Perkovic V, Agarwal R, Baldassarre J, Bakris G, Charytan DM, et al. Evaluating the effects of canagliflozin on cardiovascular and renal events in patients with type 2 diabetes mellitus and chronic kidney disease according to baseline HbA1c, including those with HbA1c <7%: results from the CREDENCE Trial. Circulation 2020;141(5):407-10. - PubMed
Chacra 2017 {published data only}
-
- Chacra A, Gantz I, Mendizabal G, Durlach L, O'Neill EA, Zimmer Z, et al. A randomised, double-blind, trial of the safety and efficacy of omarigliptin (a once-weekly DPP-4 inhibitor) in subjects with type 2 diabetes and renal impairment. International Journal of Clinical Practice 2017;71(6):e12955. - PMC - PubMed
Damman 2020 {published data only}
-
- Damman K, Beusekamp JC, Boorsma EM, Swart HP, Smilde TDJ, Elvan A, et al. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). European Journal of Heart Failure 2020;22(4):713-22. - PubMed
Davies 2016 {published data only}
-
- Davies MJ, Bain SC, Atkin SL, Rossing P, Scott D, Shamkhalova MS, et al. Efficacy and safety of liraglutide versus placebo as add-on to glucose-lowering therapy in patients with type 2 diabetes and moderate renal impairment (LIRA-RENAL): a randomized clinical trial. Diabetes Care 2016;39(2):222-30. - PubMed
Dejgaard 2016 {published data only}
-
- Dejgaard TF, Johansen NB, Frandsen CS, Asmar A, Tarnow L, Knop FK, et al. Cardiovascular effects of liraglutide in patients with type 1 diabetes: a randomised, double-blinded placebo-controlled trial (Lira-1). In: Diabetologia. Vol. Conference: 52nd Annual Meeting of the European Association for the Study of Diabetes, EASD 2016. Germany. Conference Start: 2016-09-12. Conference End: 2016-09-16. 59. 2016:S356‐S357.
Doupis 2019 {published data only}
-
- Doupis J. Once-weekly dulaglutide and major cardiovascular events: Results of the REWIND trial. US Endocrinology 2019;15(2):65-7.
Ejiri 2019 {published data only}
-
- Ejiri K, Miyoshi T, Nakamura K, Sakuragi S, Munemasa M, Namba S, et al. The effect of luseogliflozin and alpha-glucosidase inhibitor on heart failure with preserved ejection fraction in diabetic patients: Rationale and design of the MUSCAT-HF randomised controlled trial. BMJ Open 2019;9(3):e026590. - PMC - PubMed
Ferdinand 2019 {published data only}
-
- Ferdinand KC, Izzo JL, Lee J, Meng L, George J, Salsali A, et al. Antihyperglycemic and blood pressure effects of empagliflozin in black patients with type 2 diabetes mellitus and hypertension. Circulation 2019;139(18):2098-109. - PubMed
Furtado 2019a {published data only}
-
- Furtado RHM, Bonaca MP, Raz I, Zelniker TA, Mosenzon O, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes mellitus and previous myocardial infarction. Circulation 2019;139(22):2516-27. - PubMed
Furtado 2019b {published data only}
-
- Furtado RHM, Bonaca MP, Raz I, Zelniker TA, Mosenzon O, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes and prior myocardial infarction: a sub-analysis from DECLARE TIMI-58 Trial. Journal of the American College of Cardiology 2019;73(9):1.
Furuhashi 2020 {published data only}
-
- Furuhashi M, Sakuma I, Morimoto T, Higashiura Y, Sakai A, Matsumoto M, et al. Treatment with anagliptin, a DPP-4 inhibitor, decreases FABP4 concentration in patients with type 2 diabetes mellitus at a high risk for cardiovascular disease who are receiving statin therapy. Cardiovascular Diabetology 2020;19(1):89. - PMC - PubMed
Gallwitz 2012 {published data only}
-
- Gallwitz B, Rosenstock J, Rauch T, Bhattacharya S, Patel S, Eynatten M, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet 2012;380(9840):475-83. - PubMed
Gause‐Nilsson 2014 {published data only}
-
- Gause-Nilsson IAM, Bruin TW, Sugg J, Parikh S, Johnsson E, Leiter LA. Two-year efficacy and safety of dapagliflozin for patients with type 2 diabetes mellitus and a history of cardiovascular disease. Diabetologia 2014;57(1 SUPPL 1):S325.
Gerstein 2018 {published data only}
-
- Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al, REWIND Trial Investigators. Design and baseline characteristics of participants in the Researching cardiovascular Events with a Weekly INcretin in Diabetes (REWIND) trial on the cardiovascular effects of dulaglutide. Diabetes, Obesity and Metabolism 2018;20(1):42-9. - PubMed
Gerstein 2019a {published data only}
-
- Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al, REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019;394(10193):121-30. - PubMed
Gerstein 2019b {published data only}
-
- Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al, REWIND Investigators. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet 2019;394(10193):131-8. - PubMed
Gerstein 2020 {published data only}
-
- Gerstein HC, Hart R, Colhoun HM, Diaz R, Lakshmanan M, Botros FT, et al. The effect of dulaglutide on stroke: an exploratory analysis of the REWIND trial. Lancet Diabetes & Endocrinology 2020;8(2):106-14. - PubMed
Gross 2016 {published data only}
-
- Gross L, Theiss HD, Grabmaier U, Adrion C, Mansmann U, Sohn HY, et al. Combined therapy with sitagliptin plus granulocyte-colony stimulating factor in patients with acute myocardial infarction - Long-term results of the SITAGRAMI trial. International Journal of Cardiology 2016;215:441-5. - PubMed
Haering 2015 {published data only}
-
- Haering HU, Merker L, Christiansen AV, Roux F, Salsali A, Kim G, et al. Empagliflozin as add-on to metformin plus sulphonylurea in patients with type 2 diabetes. Diabetes Research and Clinical Practice 2015;110(1):82-90. - PubMed
Hamal 2020 {published data only}
-
- Hamal S, Cherukuri L, Shaikh K, Kinninger A, Doshi J, Birudaraju D, et al. Effect of semaglutide on coronary atherosclerosis progression in patients with type II diabetes: Rationale and design of the semaglutide treatment on coronary progression trial. Coronary Artery Disease 2020;31(3):306-14. - PubMed
Haneda 2016 {published data only}
-
- Haneda M, Seino Y, Inagaki N, Kaku K, Sasaki T, Fukatsu A, et al. Influence of renal function on the 52-week efficacy and safety of the sodium glucose cotransporter 2 inhibitor luseogliflozin in Japanese patients with type 2 diabetes mellitus. Clinical Therapeutics 2016;38(1):66-88.e20. - PubMed
Heerspink 2020 {published data only}
Hulst 2020 {published data only}
Imazu 2019 {published data only}
-
- Imazu M, Nakano A, Ito S, Hamasaki T, Kitakaze M, TOPLEVEL investigators and study coordinators. Effects of teneligliptin on the progressive left ventricular diastolic dysfunction in patients with type 2 diabetes mellitus in open-label, marker-stratified randomized, parallel-group comparison, standard treatment-controlled multicenter trial (TOPLEVEL Study): rationale and study design. Cardiovascular Drugs and Therapy 2019;33(3):363-70. - PubMed
Irie 2018 {published data only}
-
- Irie Y, Katakami N, Mita T, Takahara M, Matsuoka TA, Gosho M, et al, Study of Preventive Effects of Alogliptin on Diabetic Atherosclerosis (SPEAD-A) Collaborators. Evaluation of the effect of alogliptin on tissue characteristics of the carotid wall: subanalysis of the SPEAD-A Trial. Diabetes Therapy 2018;9(1):317-29. - PMC - PubMed
Jaiswal 2015 {published data only}
-
- Jaiswal M, Martin CL, Brown MB, Callaghan B, Albers JW, Feldman EL, et al. Effects of exenatide on measures of diabetic neuropathy in subjects with type 2 diabetes: Results from an 18-month proof-of-concept open-label randomized study. Journal of Diabetes and its Complications 2015;29(8):1287-94. - PMC - PubMed
Januzzi 2017 {published data only}
-
- Januzzi JL Jr, Butler J, Jarolim P, Sattar N, Vijapurkar U, Desai M, et al. Effects of canagliflozin on cardiovascular biomarkers in older adults with type 2 diabetes. Journal of the American College of Cardiology 2017;70(6):704-12. - PubMed
Jardine 2017 {published data only}
-
- Jardine MJ, Mahaffey KW, Neal B, Agarwal R, Bakris GL, Brenner BM, et al, CREDENCE study investigators. The canagliflozin and renal endpoints in diabetes with established nephropathy clinical evaluation (CREDENCE) study rationale, design, and baseline characteristics. American Journal of Nephrology 2017;46(6):462-72. - PMC - PubMed
Jardine 2020 {published data only}
-
- Jardine MJ, Zhou Z, Mahaffey KW, Oshima M, Agarwal R, Bakris G, et al, CREDENCE Study Investigators. Renal, cardiovascular, and safety outcomes of canagliflozin by baseline kidney function: a secondary analysis of the CREDENCE randomized trial. Journal of the American Society of Nephrology 2020;31(5):1128-39. - PMC - PubMed
Kadowaki 2020 {published data only}
-
- Kadowaki T, Wang G, Rosenstock J, Yabe D, Peng Y, Kanasaki K, et al. Effect of linagliptin, a dipeptidyl peptidase-4 inhibitor, compared with the sulfonylurea glimepiride on cardiovascular outcomes in Asians with type 2 diabetes: subgroup analysis of the randomized CAROLINA trial. Diabetology International 2020;12(1):87-100. - PMC - PubMed
Kato 2019 {published data only}
-
- Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RHM, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation 2019;139(22):2528-36. - PubMed
Koyama 2018 {published data only}
-
- Koyama T, Tanaka A, Yoshida H, Oyama JI, Toyoda S, Sakuma M, et al. Comparison of the effects of linagliptin and voglibose on endothelial function in patients with type 2 diabetes and coronary artery disease: a prospective, randomized, pilot study (EFFORT). Heart and Vessels 2018;33(8):958-64. - PubMed
Li 2020 {published data only}
-
- Li B, Luo YR, Tian F, Chen YD, Tian JW, Ding Y, et al. Sitagliptin attenuates the progression of coronary atherosclerosis in patients with coronary disease and type 2 diabetes. Atherosclerosis 2020;300:10-8. - PubMed
Marx 2015 {published data only}
Matsubara 2013 {published data only}
-
- Matsubara J, Sugiyama S, Akiyama E, Iwashita S, Kurokawa H, Ohba K, et al. Dipeptidyl peptidase-4 inhibitor, sitagliptin, improves endothelial dysfunction in association with its anti-inflammatory effects in patients with coronary artery disease and uncontrolled diabetes. Circulation Journal 2013;77(5):1337-44. - PubMed
McGill 2015 {published data only}
-
- McGill JB, Yki-Järvinen H, Crowe S, Woerle HJ, Eynatten M. Combination of the dipeptidyl peptidase-4 inhibitor linagliptin with insulin-based regimens in type 2 diabetes and chronic kidney disease. Diabetes and Vascular Disease Research 2015;12(4):249-57. - PubMed
Mosenzon 2019 {published data only}
-
- Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes & Endocrinology 2019;7(8):606-17. - PubMed
Natali 2017 {published data only}
-
- Natali A, Nesti L, Fabiani I, Calogero E, Di Bello V. Impact of empagliflozin on subclinical left ventricular dysfunctions and on the mechanisms involved in myocardial disease progression in type 2 diabetes: rationale and design of the EMPA-HEART trial. Cardiovascular Diabetology 2017;16(1):130. - PMC - PubMed
Nauck 2016 {published data only}
-
- Nauck MA, di Domenico M, Patel S, Kobe M, Toorawa R, Woerle HJ. Linagliptin and pioglitazone combination therapy versus monotherapy with linagliptin or pioglitazone: A randomised, double-blind, parallel-group, multinational clinical trial. Diabetes and Vascular Disease Research 2016;13(4):286-98. - PubMed
Oe 2015 {published data only}
-
- Oe H, Nakamura K, Kihara H, Shimada K, Fukuda S, Takagi T, et al, FESC, for Effect of a DPP-4 inhibitor on left ventricular diastolic dysfunction in patients with type 2 diabetes and diabetic cardiomyopathy (3D) study investigators. Comparison of effects of sitagliptin and voglibose on left ventricular diastolic dysfunction in patients with type 2 diabetes: results of the 3D trial. Cardiovascular Diabetology 2015;14:83. - PMC - PubMed
Otterbeck 2011 {published data only}
-
- Otterbeck PE, Banerji MA. The efficacy and safety of vildagliptin in the GALIANT trial: chronic kidney disease and other applications. Expert Review of Endocrinology & Metabolism 2011;6(2):143-51. - PubMed
Paiman 2020 {published data only}
-
- Paiman EHM, Eyk HJ, Aalst MMA, Bizino MB, Geest RJ, Westenberg JJM, et al. Effect of liraglutide on cardiovascular function and myocardial tissue characteristics in type 2 diabetes patients of South Asian descent living in the Netherlands: a double‐blind, randomized, placebo‐controlled trial. Journal of Magnetic Resonance Imaging 2020;51(6):1679-88. - PMC - PubMed
Perkovic 2019 {published data only}
-
- Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al, CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. New England Journal of Medicine 2019;380(24):2295-306. - PubMed
Pi‐Sunyer 2015 {published data only}
-
- Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al, SCALE Obesity and Prediabetes NN8022-1839 Study Group. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. New England Journal of Medicine 2015;373(1):11-22. - PubMed
Pratley 2019 {published data only}
-
- Pratley RE, Emerson SS, Franek E, Gilbert MP, Marso SP, McGuire DK, et al, DEVOTE Study Group. Cardiovascular safety and lower severe hypoglycaemia of insulin degludec versus insulin glargine U100 in patients with type 2 diabetes aged 65 years or older: Results from DEVOTE (DEVOTE 7). Diabetes, Obesity and Metabolism 2019;21(7):1625-33. - PMC - PubMed
Rodbard 2017 {published data only}
-
- Rodbard HW, Bode BW, Harris SB, Rose L, Lehmann L, Jarlov H, et al, Dual Action of Liraglutide and insulin degludec (DUAL) IV trial investigators. Safety and efficacy of insulin degludec/liraglutide (IDegLira) added to sulphonylurea alone or to sulphonylurea and metformin in insulin-naive people with Type 2 diabetes: the DUAL IV trial. Diabetic Medicine 2017;34(2):189-96. - PMC - PubMed
Roos 2016 {published data only}
-
- Roos ST, Timmers L, Biesbroek PS, Nijveldt R, Kamp O, Rossum AC, et al. No benefit of additional treatment with exenatide in patients with an acute myocardial infarction. International Journal of Cardiology 2016;220:809-14. - PubMed
Rosenstock 2019a {published data only}
Tolba 2017 {published data only}
-
- Tolba MKA, El Khashab KA, Said ASA. The effects of dipeptidyl peptidase-4 inhibitors on cardiovascular disease risks in type 2 diabetes mellitus. International Journal of Pharmacy and Pharmaceutical Sciences 2017;9(1):254-9.
Tripolt 2018 {published data only}
-
- Tripolt NJ, Aberer F, Riedl R, Url J, Dimsity G, Meinitzer A, et al. Effects of linagliptin on endothelial function and postprandial lipids in coronary artery disease patients with early diabetes: a randomized, placebo-controlled, double-blind trial. Cardiovascular Diabetology 2018;17(1):71. - PMC - PubMed
Wiviott 2018 {published data only}
-
- Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. The design and rationale for the Dapagliflozin Effect on Cardiovascular Events (DECLARE)-TIMI 58 Trial. American Heart Journal 2018;200:83-89. [PMID: ] - PubMed
Wiviott 2019 {published data only}
-
- Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al, DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine 2019;380(4):347-57. [PMID: ] - PubMed
Yamada 2017 {published data only}
-
- Yamada H, Tanaka A, Kusunose K, Amano R, Matsuhisa M, Daida H, et al. Effect of sitagliptin on the echocardiographic parameters of left ventricular diastolic function in patients with type 2 diabetes: a subgroup analysis of the PROLOGUE study. Cardiovascular Diabetology 2017;16(1):63. [PMID: ] - PMC - PubMed
Zinman 2019 {published data only}
-
- Zinman B, Bhosekar V, Busch R, Holst I, Ludvik B, Thielke D, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes and Endocrinology 2019;7(5):356-67. [PMID: ] - PubMed
References to studies awaiting assessment
EMPA‐HEART2 {unpublished data only}
-
- Empagliflozin and cardiac remodelling in people without diabetes. clinicaltrials.gov/ct2/show/NCT04461041 (first posted 8 July 2020).
EXCEED {unpublished data only}
-
- Examination for cardiac function effect by echocardiography in diabetes with chronic heart failure. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000027095 (date of registration 1 Jul 2017).
SUPERIOR {unpublished data only}
-
- Sitagliptin utilization in diabetic patients with coronary artery disease for improving cardiovascular outcomes (SUPERIOR study). www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000011894 (date of registration 27 September 2013).
References to ongoing studies
CANONICAL {unpublished data only}
-
- Canagliflozin heart failure with preserved ejection fraction study in type 2 diabetes mellitus. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000028668 (date of registration 15 August 2017).
-
- CANONICAL study. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-jRCTs051180030 (date of regisration 24 January 2019).
DAPPER {published data only}
-
- DAPPER study. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-jRCTs051180135 (date of registration 18 March 2019).
-
- Yoshihara F, Imazu M, Hamasaki T, Anzai T, Yasuda S, Ito S, et al, DAPPER investigators and study coordinators. An exploratory study of dapagliflozin for the attenuation of albuminuria in patients with heart failure and type 2 diabetes mellitus (DAPPER). Cardiovascular Drugs and Therapy 2018;32(2):183-90. [PMID: ] - PubMed
DELIVER {unpublished data only}
-
- Dapagliflozin evaluation to improve the lives of patients With preserved ejection fraction heart failure. clinicaltrials.gov/show/NCT03619213 (first posted 7 August 2018).
-
- DELIVER. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-JapicCTI-184157 (date of registration 17 October 2018).
-
- Study to evaluate if dapagliflozin treatment is effective in patients with heart failure with preserved ejection fraction in reducing the risk of cardiovascular death and hospitalizations/urgent outpatient visits due to worsening heart failure. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2018-000802-46-PL (date of registration 18 September 2018).
EMMY {published data only}
-
- Impact of empagliflozin on heart in patients with myocardial infarction. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2016-004591-22-AT (date of registration 7 February 2017).
-
- Tripolt NJ, Kolesnik E, Pferschy PN, Verheyen N, Ablasser K, Sailer S, et al, EMMY study group. Impact of EMpagliflozin on cardiac function and biomarkers of heart failure in patients with acute MYocardial infarction - the EMMY trial. American Heart Journal 2020;221:39-47. [PMID: ] - PubMed
EMPA‐TROPISM {published data only}
-
- Santos-Gallego CG, Garcia-Ropero A, Mancini D, Pinney SP, Contreras JP, Fergus I, et al. Rationale and design of the EMPA-TROPISM Trial (ATRU-4): are the "cardiac benefits" of empagliflozin independent of its hypoglycemic activity? Cardiovascular Drugs and Therapy 2019;33(1):87-95. [PMID: ] - PubMed
EMPEROR‐Preserved {published data only}
-
- Anker SD, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, et al, EMPEROR-Preserved Trial Committees and Investigators. Evaluation of the effects of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: rationale for and design of the EMPEROR-Preserved Trial. European Journal of Heart Failure 2019;21(10):1279-87. [PMID: ] - PubMed
-
- Anker SD, Zannad F, Butler J, Filippatos G, Salsali A, Kimura K, et al. Design and rationale of the EMPEROR trials of, empagliflozin 10 mg once daily, in patients with chronic heart failure with reduced ejection fraction (EMPEROR-Reduced) or preserved ejection fraction (EMPEROR-Preserved). Diabetologie und Stoffwechsel 2019;14(S 01):S66.
-
- Empagliflozin outcome trial in patients with chronic heart failure (EMPEROR-Preserved). www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2016-002278-11-BE (date of registration 21 March 2018).
-
- Empagliflozin outcome trial in patients with chronic heart failure with preserved ejection fraction (EMPEROR-Preserved). clinicaltrials.gov/show/NCT03057951 (first posted 20 February 2017).
LEADPACE {unpublished data only}
-
- Efficacy and safety of liraglutide in type 2 diabetes with lower extremity arterial disease. clinicaltrials.gov/show/NCT04146155 (first posted 31 October 2019).
Li 2018 {published data only}
-
- Effects of a dipeptidyl peptidase-4 Inhibitor sitagliptininsulin on the progression of coronary atherosclerosis in patients with type 2 diabetes. clinicaltrials.gov/ct2/show/NCT03602638 (first posted 27 July 2018). [NCT: 03602638]
MEASURE‐HF {unpublished data only}
-
- A 24-week study to investigate the effects of saxagliptin,saxagliptin combined with dapagliflozin, sitagliptin and placebo in patients with type 2 diabetes mellitus and heart failure. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2015-004825-14-ES (date of registration 18 March 2016).
-
- Mechanistic evaluation of glucose-lowering strategies in patients with heart failure. clinicaltrials.gov/show/NCT02917031 (first posted 28 September 2016).
REFORM {published and unpublished data}
-
- The REFORM Trial. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2014-002742-42-GB (date of registration 9 July 2015).
SELECT {unpublished data only}
-
- SELECT - Semaglutide effects on heart disease and stroke in patients with overweight or obesity. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2017-003380-35-AT (date of registration 13 December 2018).
-
- Semaglutide effects on heart disease and stroke in patients with overweight or obesity. clinicaltrials.gov/show/NCT03574597 (first posted 2 July 2018).
STEXAS {published data only}
SUGAR‐DM‐HF {unpublished data only}
-
- Studying effects of medication (empagliflozin) in diabetes and heart failure. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2016-003719-37-GB (date of registration 9 May 2018).
Additional references
ADA 2018
-
- American Diabetes Association. Standards of medical care in diabetes - 2018. Diabetes Care 2018;42:S1-S159.
ADA 2019
-
- American Diabetes Association. Standards of medical care in diabetes - 2019. Diabetes Care 2019;42:S1-S193. - PubMed
Ambrosy 2014
-
- Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. Journal of the American College of Cardiology 2014;63(12):1123-33. [PMID: ] - PubMed
Bairey Merz 2019
-
- Bairey Merz CN. Testing for coronary microvascular dysfunction. JAMA 2019;322(23):2358. - PubMed
Ceriello 2011
Clar 2012
Conrad 2018
Deeks 2020
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
DPP Research Group 2012
Egger 1997
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 6 December 2020. Available at gradepro.org.
Heerspink 2017
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Hong 2013
IDF 2019
-
- International Diabetes Federation. DF Diabetes Atlas, 9th edn. Brussels, Belgium: 2019. Available from www.diabetesatlas.org/en.
Ingelsson 2005
-
- Ingelsson E, Sundstrom J, Arnlov J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. JAMA 2005;294(3):334-41. [PMID: ] - PubMed
Jadhav 2006
-
- Jadhav S, Ferrell W, Greer IA, Petrie JR, Cobbe SM, Sattar N. Effects of metformin on microvascular function and exercise tolerance in women with angina and normal coronary arteries: a randomized, double-blind, placebo-controlled study. Journal of the American College of Cardiology 2006;48(5):956-63. [PMID: ] - PubMed
Joseph 2017
-
- Joseph P, Leong D, McKee M, Anand SS, Schwalm JD, Teo K, et al. Reducing the global burden of cardiovascular disease, part 1: the epidemiology and risk factors. Circulation Research 2017;121(6):677-94. - PubMed
Kim 2019
-
- Kim J, Park S, Kim H, Je NK. National trends in metformin-based combination therapy of oral hypoglycaemic agents for type 2 diabetes mellitus. European Journal of Clinical Pharmacology 2019;75(12):1723-30. - PubMed
Koliaki 2011
Lefebvre 2020
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Li 2016a
Li 2016b
Liberati 2009
Luo 2019
Maggioni 2015
-
- Maggioni AP. Epidemiology of heart failure in Europe. Heart Failure Clinics 2015;11(4):625-35. [PMID: ] - PubMed
McGuire 2021
Mclntyre 1991
-
- McIntyre HD, Ma A, Bird DM, Paterson CA, Ravenscroft PJ, Cameron DP. Metformin increases insulin sensitivity and basal glucose clearance in type 2 (non-insulin dependent) diabetes mellitus. Australian and New Zealand Journal of Medicine 1991;21(5):714-9. [PMID: ] - PubMed
Mensah 2017
Nikolakopoulou 2020
Owan 2005
-
- Owan TE, Redfield MM. Epidemiology of diastolic heart failure. Progress in Cardiovascular Diseases 2005;47(5):320-32. [PMID: ] - PubMed
Ponikowski 2014
-
- Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, et al. Heart failure: preventing disease and death worldwide. ESC Heart Failure 2014;1(1):4-25. [PMID: ] - PubMed
Ponikowski 2016
-
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al, ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). European Heart Journal 2016;37(27):2129-200. [PMID: ] - PubMed
Puhan 2014
-
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al, GRADE Working Group. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014;349:g5630. [PMID: ] - PubMed
Pulakazhi 2019
-
- Pulakazhi Venu VK, El-Daly M, Saifeddine M, Hirota SA, Ding H, Triggle CR, et al. Minimizing hyperglycemia-induced vascular endothelial dysfunction by inhibiting endothelial sodium-glucose cotransporter 2 and attenuating oxidative stress: implications for treating individuals with type 2 diabetes. Canadian Journal of Diabetes 2019;43(7):510-4. [PMID: ] - PubMed
R 2017 [Computer program]
-
- R: a language and environment for statistical computing. Version 3.4.2. Vienna, Austria: R Foundation for Statistical Computing, 2017. Available at www.R-project.org.
Rena 2017
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Roth 2015a
-
- Roth GA, Huffman MD, Moran AE, Feigin V, Mensah GA, Naghavi M, et al. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation 2015;132(17):1667-78. [PMID: ] - PubMed
Roth 2015b
Sato 2015
-
- Sato N. Epidemiology of heart failure in Asia. Heart Failure Clinics 2015;11(4):573-9. [PMID: ] - PubMed
Savarese 2016
-
- Savarese G, D'Amore C, Federici M, De Martino F, Dellegrottaglie S, Marciano C, et al. Effects of dipeptidyl peptidase 4 inhibitors and sodium-glucose linked co-transporter-2 inhibitors on cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis. International Journal of Cardiology 2016;220:595-601. [PMID: ] - PubMed
Schünemann 2020
-
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Selvin 2004
-
- Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Annals of Internal Medicine 2004;141(6):421-31. [PMID: ] - PubMed
Sheikh 2013
Shimokawa 2015
-
- Shimokawa H, Miura M, Nochioka K, Sakata Y. Heart failure as a general pandemic in Asia. European Journal of Heart Failure 2015;17(9):884-92. [PMID: ] - PubMed
Tanabe 2015
UKPDS 1998
-
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352(9139):1558. [PMID: ] - PubMed
Vilsbøll 2001
-
- Vilsbøll T, Krarup T, Deacon CF, Madsbad S, Holst JJ. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes 2001;50(3):609-13. [PMID: ] - PubMed
White 2014
Wu 2016
-
- Wu JH, Foote C, Blomster J, Toyama T, Perkovic V, Sundström J, et al. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes & Endocrinology 2016;4(5):411-9. [PMID: ] - PubMed
Yepes‐Nuñez 2019
-
- Yepes-Nuñez JJ, Li SA, Guyatt G, Jack SM, Brozek JL, Beyene J, et al. Development of the summary of findings table for network meta-analysis. Journal of Clinical Epidemiology 2019;115:1-13. [PMID: ] - PubMed
Zelniker 2019
-
- Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019;393(10166):31-9. [PMID: ] - PubMed
Zhang 2018
-
- Zhang XL, Zhu QQ, Chen YH, Li XL, Chen F, Huang JA, Xu B. Cardiovascular safety, long-term noncardiovascular safety, and efficacy of sodium-glucose cotransporter 2 inhibitors in patients with type 2 diabetes mellitus: a systemic review and meta-analysis with trial sequential analysis. Journal of the American Heart Association 2018;7(2):e007165. [DOI: 10.1161/JAHA.117.007165] [PMID: ] - DOI - PMC - PubMed
Zheng 2018
-
- Zheng SL, Roddick AJ, Aghar-Jaffar R, Shun-Shin MJ, Francis D, Oliver N, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA 2018;319(15):1580-91. [PMID: ] - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

