Cardiac Investigations in Sudden Unexpected Death in DEPDC5-Related Epilepsy
- PMID: 34693554
- PMCID: PMC9299146
- DOI: 10.1002/ana.26256
Cardiac Investigations in Sudden Unexpected Death in DEPDC5-Related Epilepsy
Abstract
Objective: Germline loss-of-function mutations in DEPDC5, and in its binding partners (NPRL2/3) of the mammalian target of rapamycin (mTOR) repressor GATOR1 complex, cause focal epilepsies and increase the risk of sudden unexpected death in epilepsy (SUDEP). Here, we asked whether DEPDC5 haploinsufficiency predisposes to primary cardiac defects that could contribute to SUDEP and therefore impact the clinical management of patients at high risk of SUDEP.
Methods: Clinical cardiac investigations were performed in 16 patients with pathogenic variants in DEPDC5, NPRL2, or NPRL3. Two novel Depdc5 mouse strains, a human HA-tagged Depdc5 strain and a Depdc5 heterozygous knockout with a neuron-specific deletion of the second allele (Depdc5c/- ), were generated to investigate the role of Depdc5 in SUDEP and cardiac activity during seizures.
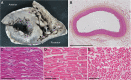
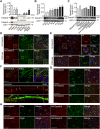
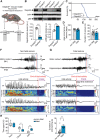
Results: Holter, echocardiographic, and electrocardiographic (ECG) examinations provided no evidence for altered clinical cardiac function in the patient cohort, of whom 3 DEPDC5 patients succumbed to SUDEP and 6 had a family history of SUDEP. There was no cardiac injury at autopsy in a postmortem DEPDC5 SUDEP case. The HA-tagged Depdc5 mouse revealed expression of Depdc5 in the brain, heart, and lungs. Simultaneous electroencephalographic-ECG records on Depdc5c/- mice showed that spontaneous epileptic seizures resulting in a SUDEP-like event are not preceded by cardiac arrhythmia.
Interpretation: Mouse and human data show neither structural nor functional cardiac damage that might underlie a primary contribution to SUDEP in the spectrum of DEPDC5-related epilepsies. ANN NEUROL 2022;91:101-116.
© 2021 The Authors. Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Nothing to report.
Figures


References
-
- Nashef L, So EL, Ryvlin P, Tomson T. Unifying the definitions of sudden unexpected death in epilepsy. Epilepsia 2012;53:227–233. - PubMed
-
- Harden C, Tomson T, Gloss D, et al. Practice guideline summary: sudden unexpected death in epilepsy incidence rates and risk factors: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsy Curr 2017;17:180–187. - PMC - PubMed
-
- Whitney R, Donner EJ. Risk factors for sudden unexpected death in epilepsy (SUDEP) and their mitigation. Curr Treat Options Neurol 2019;21:7. - PubMed
-
- Ryvlin P, Nashef L, Lhatoo SD, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol 2013;12:966–977. - PubMed
-
- Devinsky O, Hesdorffer DC, Thurman DJ, et al. Sudden unexpected death in epilepsy: epidemiology, mechanisms, and prevention. Lancet Neurol 2016;15:1075–1088. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

