A new view of macula densa cell protein synthesis
- PMID: 34693742
- PMCID: PMC8714974
- DOI: 10.1152/ajprenal.00222.2021
A new view of macula densa cell protein synthesis
Abstract
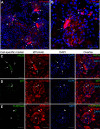
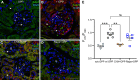
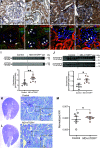
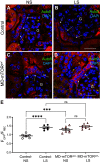
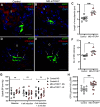
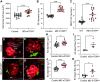
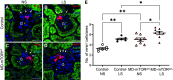
Macula densa (MD) cells, a chief sensory cell type in the nephron, are endowed with unique microanatomic features including a high density of protein synthetic organelles and secretory vesicles in basal cell processes ("maculapodia") that suggest a so far unknown high rate of MD protein synthesis. This study aimed to explore the rate and regulation of MD protein synthesis and their effects on glomerular function using novel transgenic mouse models, newly established fluorescence cell biology techniques, and intravital microscopy. Sox2-tdTomato kidney tissue sections and an O-propargyl puromycin incorporation-based fluorescence imaging assay showed that MD cells have the highest level of protein synthesis within the kidney cortex followed by intercalated cells and podocytes. Genetic gain of function of mammalian target of rapamycin (mTOR) signaling specifically in MD cells (in MD-mTORgof mice) or their physiological activation by low-salt diet resulted in further significant increases in the synthesis of MD proteins. Specifically, these included both classic and recently identified MD-specific proteins such as cyclooxygenase 2, microsomal prostaglandin E2 synthase 1, and pappalysin 2. Intravital imaging of the kidney using multiphoton microscopy showed significant increases in afferent and efferent arteriole and glomerular capillary diameters and blood flow in MD-mTORgof mice coupled with an elevated glomerular filtration rate. The presently identified high rate of MD protein synthesis that is regulated by mTOR signaling is a novel component of the physiological activation and glomerular hemodynamic regulatory functions of MD cells that remains to be fully characterized.NEW & NOTEWORTHY This study discovered the high rate of protein synthesis in macula densa (MD) cells by applying direct imaging techniques with single cell resolution. Physiological activation and mammalian target of rapamycin signaling played important regulatory roles in this process. This new feature is a novel component of the tubuloglomerular cross talk and glomerular hemodynamic regulatory functions of MD cells. Future work is needed to elucidate the nature and (patho)physiological role of the specific proteins synthesized by MD cells.
Keywords: glomerular filtration rate; mTOR; macula densa; protein synthesis; renin.
Conflict of interest statement
J.P-P. and G.G. are cofounders of Macula Densa Cell LLC, a biotechnology company that develops therapeutics to target macula densa cells for a regenerative treatment for chronic kidney disease. Macula Densa Cell LLC has a patent entitled “Targeting macula densa cells as a new therapeutic approach for kidney disease.” J.P-P. received consulting fees from Travere Therapeutics and Eli Lilly & Co. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures


Similar articles
-
A new view of macula densa cell microanatomy.Am J Physiol Renal Physiol. 2021 Mar 1;320(3):F492-F504. doi: 10.1152/ajprenal.00546.2020. Epub 2021 Jan 25. Am J Physiol Renal Physiol. 2021. PMID: 33491562 Free PMC article.
-
The macula densa prorenin receptor is essential in renin release and blood pressure control.Am J Physiol Renal Physiol. 2018 Sep 1;315(3):F521-F534. doi: 10.1152/ajprenal.00029.2018. Epub 2018 Apr 18. Am J Physiol Renal Physiol. 2018. PMID: 29667908 Free PMC article.
-
Activation of the succinate receptor GPR91 in macula densa cells causes renin release.J Am Soc Nephrol. 2009 May;20(5):1002-11. doi: 10.1681/ASN.2008070740. Epub 2009 Apr 23. J Am Soc Nephrol. 2009. PMID: 19389848 Free PMC article.
-
Role of macula densa neuronal nitric oxide synthase in renal diseases.Med Mol Morphol. 2006 Mar;39(1):2-7. doi: 10.1007/s00795-006-0310-2. Med Mol Morphol. 2006. PMID: 16575507 Review.
-
Macula densa neuronal nitric oxide synthase.Cardiovasc Res. 2002 Nov;56(2):189-96. doi: 10.1016/s0008-6363(02)00536-9. Cardiovasc Res. 2002. PMID: 12393089 Review.
Cited by
-
ZO-1 expression in normal human macula densa: Immunohistochemical and immunofluorescence investigations.J Anat. 2023 Jun;242(6):1184-1188. doi: 10.1111/joa.13832. Epub 2023 Jan 31. J Anat. 2023. PMID: 36719664 Free PMC article.
-
Intravital Multiphoton Microscopy as a Tool for Studying Renal Physiology, Pathophysiology and Therapeutics.Front Physiol. 2022 Mar 24;13:827280. doi: 10.3389/fphys.2022.827280. eCollection 2022. Front Physiol. 2022. PMID: 35399274 Free PMC article. Review.
-
See the power in kidney cells with ATP biosensor.Kidney Int. 2024 Sep;106(3):362-364. doi: 10.1016/j.kint.2024.06.019. Kidney Int. 2024. PMID: 39174197
-
Ion channels and channelopathies in glomeruli.Physiol Rev. 2023 Jan 1;103(1):787-854. doi: 10.1152/physrev.00013.2022. Epub 2022 Aug 25. Physiol Rev. 2023. PMID: 36007181 Free PMC article. Review.
-
Developmental origins of disease - Effects of iron deficiency in the rat developing kidney and beyond.Pediatr Nephrol. 2025 Apr 12. doi: 10.1007/s00467-025-06762-w. Online ahead of print. Pediatr Nephrol. 2025. PMID: 40220077 Review.
References
-
- Yu A, Chertow G, Luyckx V, Marsden P, Skorecki K, Taal M (Editors). Anatomy of the kidney. In: Brenner and Rector's The Kidney E-Book. Amsterdam, The Netherlands: Elsevier Health Sciences, 2015, p. 42–82.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

