The Feline Cardiomyopathies: 2. Hypertrophic cardiomyopathy
- PMID: 34693811
- PMCID: PMC8642168
- DOI: 10.1177/1098612X211020162
The Feline Cardiomyopathies: 2. Hypertrophic cardiomyopathy
Abstract
Practical relevance: Hypertrophic cardiomyopathy (HCM) is the most common form of feline cardiomyopathy observed clinically and may affect up to approximately 15% of the domestic cat population, primarily as a subclinical disease. Fortunately, severe HCM, leading to heart failure or arterial thromboembolism (ATE), only occurs in a small proportion of these cats.
Patient group: Domestic cats of any age from 3 months upward, of either sex and of any breed, can be affected. A higher prevalence in male and domestic shorthair cats has been reported.
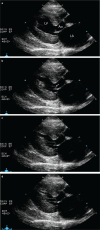
Diagnostics: Subclinical feline HCM may or may not produce a heart murmur or gallop sound. Substantial left atrial enlargement can often be identified radiographically in cats with severe HCM. Biomarkers should not be relied on solely to diagnose the disease. While severe feline HCM can usually be diagnosed via echocardiography alone, feline HCM with mild to moderate left ventricular (LV) wall thickening is a diagnosis of exclusion, which means there is no definitive test for HCM in these cats and so other disorders that can cause mild to moderate LV wall thickening (eg, hyperthyroidism, systemic hypertension, acromegaly, dehydration) need to be ruled out.
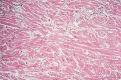
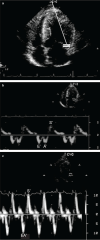
Key findings: While a genetic cause of HCM has been identified in two breeds and is suspected in another, for most cats the cause is unknown. Systolic anterior motion of the mitral valve (SAM) is the most common cause of dynamic left ventricular outflow tract obstruction (DLVOTO) and, in turn, the most common cause of a heart murmur with feline HCM. While severe DLVOTO is probably clinically significant and so should be treated, lesser degrees probably are not. Furthermore, since SAM can likely be induced in most cats with HCM, the distinction between HCM without obstruction and HCM with obstruction (HOCM) is of limited importance in cats. Diastolic dysfunction, and its consequences of abnormally increased atrial pressure leading to signs of heart failure, and sluggish atrial blood flow leading to ATE, is the primary abnormality that causes clinical signs and death in affected cats. Treatment (eg, loop diuretics) is aimed at controlling heart failure. Preventive treatment (eg, antithrombotic drugs) is aimed at reducing the risk of complications (eg, ATE).
Conclusions: Most cats with HCM show no overt clinical signs and live a normal or near-normal life despite this disease. However, a substantial minority of cats develop overt clinical signs referable to heart failure or ATE that require treatment. For most cats with clinical signs caused by HCM, the long-term prognosis is poor to grave despite therapy.
Areas of uncertainty: Genetic mutations (variants) that cause HCM have been identified in a few breeds, but, despite valiant efforts, the cause of HCM in the vast majority of cats remains unknown. No treatment currently exists that reverses or even slows the cardiomyopathic process in HCM, again despite valiant efforts. The search goes on.
Keywords: Cardiomyopathies; echocardiography; gene mutation; hypertrophic cardiomyopathy; mitral valve; myocardial diseases; systolic anterior motion.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and / or publication of this article.
Figures













References
-
- Cirino AL, Ho C. Hypertrophic cardiomyopathy overview. In: Adam MP, Ardinger HH, Pagon RA, et al. (eds). GeneReviews®. Seattle, WA: University of Washington, Seattle, 1993. Available at: http://www.ncbi.nlm.nih.gov/books/NBK1768/. - PubMed
-
- Ommen SR, Mital S, Burke MA, et al.. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hyper-trophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. ] Am Coll Cardiol 2020; 76: e159–240. - PubMed
-
- Maron BJ. Clinical course and management of hypertrophic cardiomyopathy. N Engl ] Med 2018; 379: 655–668 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

