Novel chimeric monoclonal antibodies that block fentanyl effects and alter fentanyl biodistribution in mice
- PMID: 34693882
- PMCID: PMC8547829
- DOI: 10.1080/19420862.2021.1991552
Novel chimeric monoclonal antibodies that block fentanyl effects and alter fentanyl biodistribution in mice
Abstract
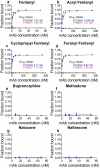
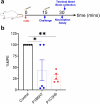
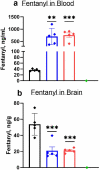
The prevalence and societal impact of opioid use disorder (OUD) is an acknowledged public health crisis that is further aggravated by the current pandemic. One of the devastating consequences of OUD is opioid overdose deaths. While multiple medications are now available to treat OUD, given the prevalence and societal burden, additional well-tolerated and effective therapies are still needed. To this point, we have developed chimeric monoclonal antibodies (mAb) that will specifically complex with fentanyl and its analogs in the periphery, thereby preventing them from reaching the central nervous system. Additionally, mAb-based passive immunotherapy offers a high degree of specificity to drugs of abuse and does not interfere with an individual's ability to use any of the medications used to treat OUD. We hypothesized that sequestering fentanyl and its analogs in the periphery will mitigate their negative effects on the brain and peripheral organs. This study is the first report of chimeric mAb against fentanyl and its analogs. We have discovered, engineered the chimeric versions, and identified the selectivity of these antibodies, through in vitro characterization and in vivo animal challenge studies. Two mAb candidates with very high (0.1-1.3 nM) binding affinities to fentanyl and its analogs were found to be effective in engaging fentanyl in the periphery and blocking its effects in challenged animals. Results presented in this work constitute a major contribution in the field of novel therapeutics targeting OUD.
Keywords: Fentanyl mAb; fentanyl analogs; opioid use disorder; opioids; passive immunization.
Conflict of interest statement
This material has been reviewed by the Walter Reed Army Institute of Research, the National Institute on Drug Abuse, and Indiana Biosciences Research Institute. All animal studies were conducted under an approved animal use protocol in an Association for Assessment and Accreditation of Laboratory Animal Care International-accredited facility in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals. Experiments involving animals adhered to the principles stated in the Guide for the Care and Use of Laboratory Animals, 8th edition. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the authors, and should not be construed as official, or as reflecting true views of the Department of the Army, the Department of Defense, NIDA, NIH or the US government. GRM, AS, KCR, and AEJ are inventors of a provision patent application filed by the Henry M. Jackson Foundation for the Advancement of Military Medicine (provisional patent Serial No.: 62/960,187; January 13, 2020).
Figures



References
-
- National Vital Statistics System . 2019. Accessed: March 1, 2021. https://www.cdc.gov/nchs/nvss/index.htm
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
