Cardiovascular Progerin Suppression and Lamin A Restoration Rescue Hutchinson-Gilford Progeria Syndrome
- PMID: 34694158
- PMCID: PMC8614561
- DOI: 10.1161/CIRCULATIONAHA.121.055313
Cardiovascular Progerin Suppression and Lamin A Restoration Rescue Hutchinson-Gilford Progeria Syndrome
Abstract
Background: Hutchinson-Gilford progeria syndrome (HGPS) is a rare disorder characterized by premature aging and death mainly because of myocardial infarction, stroke, or heart failure. The disease is provoked by progerin, a variant of lamin A expressed in most differentiated cells. Patients look healthy at birth, and symptoms typically emerge in the first or second year of life. Assessing the reversibility of progerin-induced damage and the relative contribution of specific cell types is critical to determining the potential benefits of late treatment and to developing new therapies.
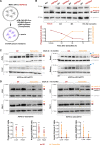
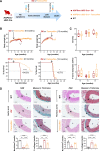
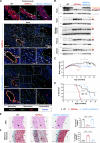
Methods: We used CRISPR-Cas9 technology to generate LmnaHGPSrev/HGPSrev (HGPSrev) mice engineered to ubiquitously express progerin while lacking lamin A and allowing progerin suppression and lamin A restoration in a time- and cell type-specific manner on Cre recombinase activation. We characterized the phenotype of HGPSrev mice and crossed them with Cre transgenic lines to assess the effects of suppressing progerin and restoring lamin A ubiquitously at different disease stages as well as specifically in vascular smooth muscle cells and cardiomyocytes.
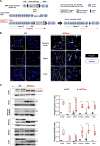
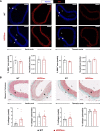
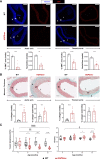
Results: Like patients with HGPS, HGPSrev mice appear healthy at birth and progressively develop HGPS symptoms, including failure to thrive, lipodystrophy, vascular smooth muscle cell loss, vascular fibrosis, electrocardiographic anomalies, and precocious death (median lifespan of 15 months versus 26 months in wild-type controls, P<0.0001). Ubiquitous progerin suppression and lamin A restoration significantly extended lifespan when induced in 6-month-old mildly symptomatic mice and even in severely ill animals aged 13 months, although the benefit was much more pronounced on early intervention (84.5% lifespan extension in mildly symptomatic mice, P<0.0001, and 6.7% in severely ill mice, P<0.01). It is remarkable that major vascular alterations were prevented and lifespan normalized in HGPSrev mice when progerin suppression and lamin A restoration were restricted to vascular smooth muscle cells and cardiomyocytes.
Conclusions: HGPSrev mice constitute a new experimental model for advancing knowledge of HGPS. Our findings suggest that it is never too late to treat HGPS, although benefit is much more pronounced when progerin is targeted in mice with mild symptoms. Despite the broad expression pattern of progerin and its deleterious effects in many organs, restricting its suppression to vascular smooth muscle cells and cardiomyocytes is sufficient to prevent vascular disease and normalize lifespan.
Keywords: Hutchinson-Gilford progeria syndrome; cardiac myocyte; cell; smooth muscle.
Figures


References
-
- Dorado B, Andrés V. A-type lamins and cardiovascular disease in premature aging syndromes. Curr Opin Cell Biol. 2017;46:17–25. doi: 10.1016/j.ceb.2016.12.005 - PubMed
-
- De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, Boccaccio I, Lyonnet S, Stewart CL, Munnich A, Le Merrer M, et al. . Lamin a truncation in Hutchinson-Gilford progeria. Science. 2003;300:2055. doi: 10.1126/science.1084125 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

