Piezo1-Pannexin1 complex couples force detection to ATP secretion in cholangiocytes
- PMID: 34694360
- PMCID: PMC8548913
- DOI: 10.1085/jgp.202112871
Piezo1-Pannexin1 complex couples force detection to ATP secretion in cholangiocytes
Abstract
Cholangiocytes actively contribute to the final composition of secreted bile. These cells are exposed to abnormal mechanical stimuli during obstructive cholestasis, which has a deep impact on their function. However, the effects of mechanical insults on cholangiocyte function are not understood. Combining gene silencing and pharmacological assays with live calcium imaging, we probed molecular candidates essential for coupling mechanical force to ATP secretion in mouse cholangiocytes. We show that Piezo1 and Pannexin1 are necessary for eliciting the downstream effects of mechanical stress. By mediating a rise in intracellular Ca2+, Piezo1 acts as a mechanosensor responsible for translating cell swelling into activation of Panx1, which triggers ATP release and subsequent signal amplification through P2X4R. Co-immunoprecipitation and pull-down assays indicated physical interaction between Piezo1 and Panx1, which leads to stable plasma membrane complexes. Piezo1-Panx1-P2X4R ATP release pathway could be reconstituted in HEK Piezo1 KO cells. Thus, our data suggest that Piezo1 and Panx1 can form a functional signaling complex that controls force-induced ATP secretion in cholangiocytes. These findings may foster the development of novel therapeutic strategies for biliary diseases.
© 2021 Desplat et al.
Figures


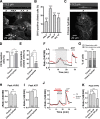



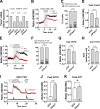

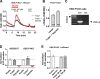



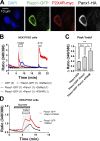

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

