Neoadjuvant immunotherapy in primary and metastatic colorectal cancer
- PMID: 34694371
- PMCID: PMC10364874
- DOI: 10.1093/bjs/znab342
Neoadjuvant immunotherapy in primary and metastatic colorectal cancer
Abstract
Background: Colorectal cancer (CRC) is the second most common solid organ cancer. Traditional treatment is with surgery and chemotherapy. Immunotherapy has recently emerged as a neoadjuvant therapy that could change treatment strategy in both primary resectable and metastatic CRC.
Methods: A literature review of PubMed with a focus on studies exploring upfront immunotherapy in operable CRC, either for primary resectable stage I-III cancers or for (potentially) operable liver metastasis.
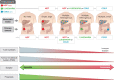
Results: Immune checkpoint blockade by the programmed cell death 1 (PD-1) receptor inhibitors nivolumab and pembrolizumab and the cytotoxic T cell-associated protein 4 (CTLA-4) inhibitor ipilimumab has shown good results in both early-stage and advanced CRC. The effects of immune checkpoint inhibitors have so far been demonstrated in small phase I/II studies and predominantly in treatment-refractory stage IV disease with defect Mismatch repair (dMMR). However, recent data from phase I/II (NICHE-1) studies suggest an upfront role for immunotherapy in operable stage I-III disease. By blocking crucial immune checkpoints, cytotoxic T cells are activated and release cytotoxic signals that initiate cancer cell destruction. The very high complete response rate in dMMR operable CRC with neoadjuvant immunotherapy with nivolumab and ipilimumab, and even partial pathological response in some patients with proficient MMR (pMMR) CRC, calls for further attention to patient selection for neoadjuvant treatment, beyond MMR status alone.
Conclusion: Early data on the effect of immunotherapy in CRC provide new strategic thinking of treatment options in CRC for both early-stage and advanced disease, with prospects for new trials.
Plain language summary
Immunotherapy has proven to be highly effective as first-line treatment of metastatic colorectal cancer (CRC). Further, immune checkpoint blockade by the programmed cell death 1 (PD-1) receptor inhibitors nivolumab and pembrolizumab and the cytotoxic T cell-associated protein 4 (CTLA-4) inhibitor ipilimumab has provided very good results in both early-stage and advanced CRC. The high response rate in dMMR in operable colon cancers by preoperative use of double nivolumab and ipilimumab therapy warrants further investigation into its impact on long-term overall survival. Hence, immunotherapy has emerged as a neoadjuvant approach, possibly changing treatment strategy for both primary resectable and metastatic CRC. Larger phase III trials are needed to evaluate overall effects on survival.
© The Author(s) 2021. Published by Oxford University Press on behalf of BJS Society Ltd.
Figures


References
-
- Messersmith WA. NCCN guidelines updates: management of metastatic colorectal cancer. J Natl Compr Canc Netw 2019;17:599–601. - PubMed
-
- Stebbing J, Singh Wasan H.. Decoding metastatic colorectal cancer to improve clinical decision making. J Clin Oncol 2019;37:1847–1850. - PubMed
-
- Dienstmann R, Vermeulen L, Guinney J, Kopetz S, Tejpar S, Tabernero J.. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer 2017;17:79–92. - PubMed
-
- Hanahan D, Weinberg RA.. Hallmarks of cancer: the next generation. Cell 2011;144:646–674. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

