The IFN-inducible GTPase IRGB10 regulates viral replication and inflammasome activation during influenza A virus infection in mice
- PMID: 34694641
- PMCID: PMC8813912
- DOI: 10.1002/eji.202149305
The IFN-inducible GTPase IRGB10 regulates viral replication and inflammasome activation during influenza A virus infection in mice
Abstract
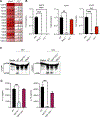
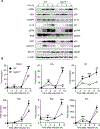
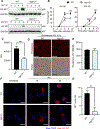
The upregulation of interferon (IFN)-inducible GTPases in response to pathogenic insults is vital to host defense against many bacterial, fungal, and viral pathogens. Several IFN-inducible GTPases play key roles in mediating inflammasome activation and providing host protection after bacterial or fungal infections, though their role in inflammasome activation after viral infection is less clear. Among the IFN-inducible GTPases, the expression of immunity-related GTPases (IRGs) varies widely across species for unknown reasons. Here, we report that IRGB10, but not IRGM1, IRGM2, or IRGM3, is required for NLRP3 inflammasome activation in response to influenza A virus (IAV) infection in mice. While IRGB10 functions to release inflammasome ligands in the context of bacterial and fungal infections, we found that IRGB10 facilitates endosomal maturation and nuclear translocation of IAV, thereby regulating viral replication. Corresponding with our in vitro results, we found that Irgb10-/- mice were more resistant to IAV-induced mortality than WT mice. The results of our study demonstrate a detrimental role of IRGB10 in host immunity in response to IAV and a novel function of IRGB10, but not IRGMs, in promoting viral translocation into the nucleus.
Keywords: GTPases; IRGB10; Inflammasome; Influenza A virus; NLRP3; Pyroptosis.
© 2021 Wiley-VCH GmbH.
Conflict of interest statement
Conflict of interests
The authors declare no commercial or financial conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

