Cytosolic localization and in vitro assembly of human de novo thymidylate synthesis complex
- PMID: 34694685
- PMCID: PMC9299187
- DOI: 10.1111/febs.16248
Cytosolic localization and in vitro assembly of human de novo thymidylate synthesis complex
Abstract
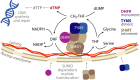
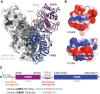
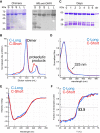
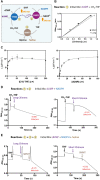
De novo thymidylate synthesis is a crucial pathway for normal and cancer cells. Deoxythymidine monophosphate (dTMP) is synthesized by the combined action of three enzymes: serine hydroxymethyltransferase (SHMT1), dihydrofolate reductase (DHFR) and thymidylate synthase (TYMS), with the latter two being targets of widely used chemotherapeutics such as antifolates and 5-fluorouracil. These proteins translocate to the nucleus after SUMOylation and are suggested to assemble in this compartment into the thymidylate synthesis complex. We report the intracellular dynamics of the complex in cancer cells by an in situ proximity ligation assay, showing that it is also detected in the cytoplasm. This result indicates that the role of the thymidylate synthesis complex assembly may go beyond dTMP synthesis. We have successfully assembled the dTMP synthesis complex in vitro, employing tetrameric SHMT1 and a bifunctional chimeric enzyme comprising human thymidylate synthase and dihydrofolate reductase. We show that the SHMT1 tetrameric state is required for efficient complex assembly, indicating that this aggregation state is evolutionarily selected in eukaryotes to optimize protein-protein interactions. Lastly, our results regarding the activity of the complete thymidylate cycle in vitro may provide a useful tool with respect to developing drugs targeting the entire complex instead of the individual components.
Keywords: cancer metabolism; protein-protein complex; purine synthesis; thymidylate synthesis; transient interactions.
© 2021 The Authors. The FEBS Journal published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

