Metathesis by Partner Interchange in σ-Bond Ligands: Expanding Applications of the σ-CAM Mechanism
- PMID: 34694734
- PMCID: PMC9299125
- DOI: 10.1002/anie.202111462
Metathesis by Partner Interchange in σ-Bond Ligands: Expanding Applications of the σ-CAM Mechanism
Abstract
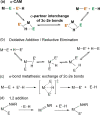
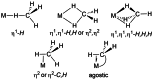
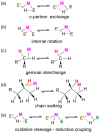
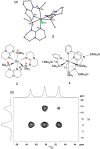
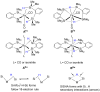
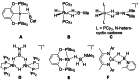
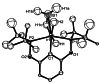
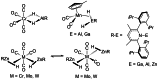
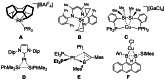
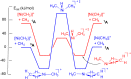
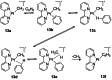
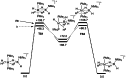
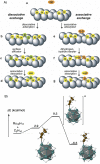
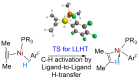
In 2007 two of us defined the σ-Complex Assisted Metathesis mechanism (Perutz and Sabo-Etienne, Angew. Chem. Int. Ed. 2007, 46, 2578-2592), that is, the σ-CAM concept. This new approach to reaction mechanisms brought together metathesis reactions involving the formation of a variety of metal-element bonds through partner-interchange of σ-bond complexes. The key concept that defines a σ-CAM process is a single transition state for metathesis that is connected by two intermediates that are σ-bond complexes while the oxidation state of the metal remains constant in precursor, intermediates and product. This mechanism is appropriate in situations where σ-bond complexes have been isolated or computed as well-defined minima. Unlike several other mechanisms, it does not define the nature of the transition state. In this review, we highlight advances in the characterization and dynamic rearrangements of σ-bond complexes, most notably alkane and zincane complexes, but also different geometries of silane and borane complexes. We set out a selection of catalytic and stoichiometric examples of the σ-CAM mechanism that are supported by strong experimental and/or computational evidence. We then draw on these examples to demonstrate that the scope of the σ-CAM mechanism has expanded to classes of reaction not envisaged in 2007 (additional σ-bond ligands, agostic complexes, sp2 -carbon, surfaces). Finally, we provide a critical comparison to alternative mechanisms for metathesis of metal-element bonds.
Keywords: agostic interactions; homogeneous catalysis; metathesis; organometallic reaction mechanisms; sigma-bond complexes.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





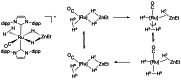

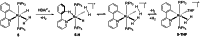
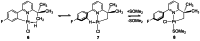
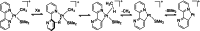
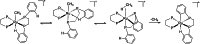



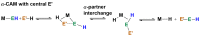

Similar articles
-
The sigma-CAM Mechanism: sigma complexes as the basis of sigma-bond metathesis at late-transition-metal centers.Angew Chem Int Ed Engl. 2007;46(15):2578-92. doi: 10.1002/anie.200603224. Angew Chem Int Ed Engl. 2007. PMID: 17380532
-
Theoretical Study of the Catalytic Hydrogenation of Alkenes by a Disilaferracyclic Complex: Can the Fe-Si σ-Bond-Assisted Activation of H-H Bonds Allow Development of a Catalysis of Iron?J Org Chem. 2016 Nov 18;81(22):10900-10911. doi: 10.1021/acs.joc.6b01961. Epub 2016 Oct 17. J Org Chem. 2016. PMID: 27704834
-
Selective Aliphatic Carbon-Carbon Bond Activation by Rhodium Porphyrin Complexes.Acc Chem Res. 2017 Jul 18;50(7):1702-1711. doi: 10.1021/acs.accounts.7b00150. Epub 2017 Jun 13. Acc Chem Res. 2017. PMID: 28609611
-
Transition metal-catalyzed carbon-carbon bond activation.Chem Soc Rev. 2004 Nov 30;33(9):610-8. doi: 10.1039/b308864m. Epub 2004 Nov 4. Chem Soc Rev. 2004. PMID: 15592626 Review.
-
Intracellular Catalysis with Selected Metal Complexes and Metallic Nanoparticles: Advances toward the Development of Catalytic Metallodrugs.Chem Rev. 2019 Jan 23;119(2):829-869. doi: 10.1021/acs.chemrev.8b00493. Epub 2019 Jan 8. Chem Rev. 2019. PMID: 30618246 Review.
Cited by
-
σ-GeH and Germyl Cationic Pt(II) Complexes.Inorg Chem. 2022 Dec 26;61(51):20848-20859. doi: 10.1021/acs.inorgchem.2c03186. Epub 2022 Nov 2. Inorg Chem. 2022. PMID: 36322561 Free PMC article.
-
On the σ-complex character of bis(gallyl)/digallane transition metal species.Chem Sci. 2023 Sep 22;14(40):11088-11095. doi: 10.1039/d3sc03772j. eCollection 2023 Oct 18. Chem Sci. 2023. PMID: 37860650 Free PMC article.
-
Activation of Si-H and B-H bonds by Lewis acidic transition metals and p-block elements: same, but different.Chem Sci. 2022 Jun 6;13(25):7392-7418. doi: 10.1039/d2sc02324e. eCollection 2022 Jun 29. Chem Sci. 2022. PMID: 35872827 Free PMC article. Review.
-
Redox-Neutral, Iron-Mediated Directed C-H Activation: General Principles and Mechanistic Insights.J Am Chem Soc. 2024 Nov 6;146(44):30637-30652. doi: 10.1021/jacs.4c12329. Epub 2024 Oct 25. J Am Chem Soc. 2024. PMID: 39450764
-
Isolobal Cationic Iridium Dihydride and Dizinc Complexes: A Dual Role for the ZnR Ligand Enhances H2 Activation.Inorg Chem. 2024 Dec 2;63(48):22944-22954. doi: 10.1021/acs.inorgchem.4c04058. Epub 2024 Nov 20. Inorg Chem. 2024. PMID: 39564932 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

