Safety and efficacy of combining biologics or small molecules for inflammatory bowel disease or immune-mediated inflammatory diseases: A European retrospective observational study
- PMID: 34694746
- PMCID: PMC8672088
- DOI: 10.1002/ueg2.12170
Safety and efficacy of combining biologics or small molecules for inflammatory bowel disease or immune-mediated inflammatory diseases: A European retrospective observational study
Abstract
Background and aims: Few data are available regarding the combination of biologics or small molecules in inflammatory bowel disease (IBD) patients. We report safety and efficacy of such combinations through a retrospective multicentre series.
Methods: Combination therapy was defined as the concomitant use of two biologics or one biologic with a small molecule. Patient demographics, disease characteristics and types of combinations were recorded. Safety was evaluated according to the occurrence of serious infection, opportunistic infection, hospitalisation, life-threatening event, worsening of IBD or immune-mediated inflammatory diseases (IMID), cancer and death. Efficacy was evaluated as the physician global assessment of the combination and comparison of clinical/endoscopic scores of IBD/IMID activity prior and during combination.
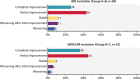
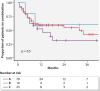
Results: A total of 104 combinations were collected in 98 patients. Concomitant IMID were present in 41 patients. Reasons for starting combination therapy were active IBD (67%), active IMID or extra-intestinal manifestations (EIM) (22%), both (10%) and unclassified in 1. Median duration of combination was 8 months (interquartile range 5-16). During 122 patient-years of follow-up, 42 significant adverse events were observed, mostly related to uncontrolled IBD. There were 10 significant infections, 1 skin cancer and no death. IBD disease activity was clinically improved in 70% and IMID/EIM activity in 81% of the patients. Overall, combination was continued in 55% of the patients.
Conclusions: Combination of biologics and small molecules in patients with IBD and IMID/EIM seems to be a promising therapeutic strategy but is also associated with a risk of opportunistic infections or infections leading to hospitalisation in 10%.
Keywords: biologics; combination therapy; immune mediated inflammatory disease; inflammatory bowel disease; safety; small molecules; treatment.
© 2021 The Authors. United European Gastroenterology Journal published by Wiley Periodicals LLC on behalf of United European Gastroenterology.
Conflict of interest statement
LG has no conflict of interest. JFC reports receiving research grants from AbbVie, Janssen Pharmaceuticals and Takeda; receiving payment for lectures from AbbVie, Amgen, Allergan, Inc. Ferring Pharmaceuticals, Shire, and Takeda; receiving consulting fees from AbbVie, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene Corporation, Celltrion, Eli Lilly, Enterome, Ferring Pharmaceuticals, Genentech, Janssen Pharmaceuticals, Landos, Ipsen, Medimmune, Merck, Novartis, Pfizer, Shire, Takeda, Tigenix, Viela bio; and hold stock options in Intestinal Biotech Development and Genfit. AO has no conflict of interest. MF reports Research grant: Amgen, Biogen, Janssen, Pfizer, Takeda. Consultancy: Abbvie, Boehringer‐Ingelheim, Janssen, MSD, Pfizer, Sandoz, Takeda. Speaker's fee: Abbvie, Amgen, Biogen, Boehringer‐Ingelheim, Falk, Ferring, Janssen, Lamepro, MSD, Mylan, Pfizer, Takeda. JS reports speaker's fees: Abbvie, Falk, and Takeda. Consultancy fees: Janssen. CJ has no conflict of interest. RS has no conflict of interest. LR reports no conflict of interest. AA reports Consultant/Advisory board: AbbVie, Allergan, Amgen, Arena Pharmaceuticals, Biogen, Bristol‐Myers Squibb, Celgene, Celltrion, Ferring, Gilead, Janssen, Lilly, Merck, Mylan, Pfizer, Roche, Samsung Bioepis, Sandoz, and Takeda; Speaker fees: AbbVie, Amgen, AstraZeneca, Biogen, Bristol‐Myers Squibb, Ferring, Gilead, Janssen, Merck, Mitsubishi Tanabe Pharma, Nikkiso, Novartis, Pfizer, Roche, Samsung Bioepis, and Takeda; Research funding: Merck, Pfizer, and Takeda. ED reports as a speaker, or has received research or education funding or advisory fees from AbbVie, Adacyte Therapeutics, Biogen, Celltrion, Gilead, Janssen, Kern Pharma, MSD, Pfizer, Roche, Samsung, Takeda, Tillots, Thermofisher. GM reports Speaker TAKEDA, Speaker MSD, speaker Jannsen, paid consultation MSD, paid consultation Genesis. AC reports Consultancy fees: Takeda, Janssen. Speaker fees: Pfizer, Abbvie. JGA has no conflict of interest. TM reports speaker's honoraria from MSD, AbbVie, Egis, Goodwill Pharma, Takeda, Pfizer and Teva. KK reports Speaker fees from Abbvie, Aenorasis, Janssen, MSD, Pfizer and Takeda Consultancy or advisory board member fees from Abbvie, Amgen, Ferring, Galenica, Genesis, Janssen, MSD, Pfizer and Takeda. KG reports KB Gecse has received grants from Pfizer Inc and Celltrion; consultancy fees from AbbVie, Arena Pharmaceuticals, Galapagos, Gilead, ImmunicTherapeutics, Janssen Pharmaceuticals, Novartis, Pfizer Inc., Samsung Bioepis and Takeda and speaker's honoraria from Celltrion, Ferring, Janssen Pharmaceuticals, Novartis, Pfizer Inc, Samsung Bioepis, Takeda and Tillotts. JVO reports speaker fees for Tillots. ML reports speaker and/or principal investigator for: Abbvie, Bristol Myers Squibb, Celgene, Covidien, Dr. Falk, Ferring Pharmaceuticals, Galapagos, Gilead, GlaxoSmithKline, Janssen‐Cilag, Merck Sharp & Dohme, Pfizer, Protagonist therapeutics, Receptos, Robarts Clinical Trials, Takeda, Tillotts, Tramedico.He has received research grants from AbbVie, Merck Sharp & Dohme, Dr Falk, Achmea healthcare, Galapagos and ZonMW. KF reports received speaker's honoraria from AbbVie, Janssen, Ferring, Takeda and Goodwill Pharma. RA reports the following paid or unpaid consultancies, business interests, or sources of honoraria payments: AbbVie, Amgen, Arena Pharmaceuticals, Biogen, Boehringer Ingelheim, Celgene, Celltrion Healthcare, Dr Falk Pharma, Ferring, Galapagos, Gilead, GlaxoSmithKline plc, InDex Pharmaceuticals, Janssen‐Cilag, Kiniksa Pharmaceuticals, MSD Sharp & Dohme, Novartis, Pandion Therapeutics, Pfizer, Roche Pharma, Samsung Bioepis, Stelic Institute & Co, Takeda Pharma, Tillotts Pharma AG, Viatris. DGR reports Paid consultancies, lecture fees for the past 2 years: Janssen, Abbvie. CS reports unrestricted research grants from Janssen and, AbbVie, has provided consultancy to Arena, Galapagos, Dr Falk, AbbVie, Takeda, Fresenius Kabi and Janssen, and had speaker arrangements with Celltrion, Dr Falk, AbbVie, Janssen, Pfizer and Takeda. FH reports advisory boards or as speaker for Abbvie, Janssen‐Cilag, MSD, Takeda, Celltrion, Teva, Sandoz and Dr Falk. Funding (Grants/Honoraria): Dr Falk, Janssen‐Cilag, Abbvie, Takeda. Consulting Fees: Celgene, Janssen‐Cilag. BB has no conflict of interest. SS reports Research grants from Takeda, AbbVie, AMGEN, Warner Chilcott, Ferring, MSD, Biohit and Cellgene, serves on the advisory boards of Takeda, AbbVie, Merck, Ferring, Pharmacocosmos, Warner Chilcott, Janssen, Falk Pharma, Biohit, TriGenix, Cellgene, Celltrion, and Tillots Pharma, and has received speaker fees from Abbvie, Jaansen, Merck, Warner Chilcott and Falk Pharma. JFR reports: Speaker fee: Abbvie, MSD, Takeda, Pfizer, Ferring, Falk, Biogen, Amgen, Celltrion. Consultancy: Abbvie, Takeda, Hospira, Mundipharma, MSD, Pfizer, GlaxoSK, Janssen. Research grand: Takeda, Abbvie.
Figures
References
-
- Glassner K, Oglat A, Duran A, Koduru P, Perry C, Wilhite A, et al. The use of combination biological or small molecule therapy in inflammatory bowel disease: a retrospective cohort study. J Dig Dis. 2020;21:264–71. - PubMed
-
- Kwapisz L, Raffals LE, Bruining DH, Pardi DS, Tremaine WJ, Kane SV, et al. Combination biologic therapy in inflammatory bowel disease: experience from a tertiary care center. Clin Gastroenterol Hepatol. 2021;19:616–17. - PubMed

