Immunotargeting of Nanocrystals by SpyCatcher Conjugation of Engineered Antibodies
- PMID: 34694776
- PMCID: PMC9035480
- DOI: 10.1021/acsnano.1c07856
Immunotargeting of Nanocrystals by SpyCatcher Conjugation of Engineered Antibodies
Abstract
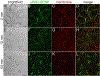
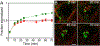
Inorganic nanocrystals such as quantum dots (QDs) and upconverting nanoparticles (UCNPs) are uniquely suited for quantitative live-cell imaging and are typically functionalized with ligands to study specific receptors or cellular targets. Antibodies (Ab) are among the most useful targeting reagents owing to their high affinities and specificities, but common nanocrystal labeling methods may orient Ab incorrectly, be reversible or denaturing, or lead to Ab-NP complexes too large for some applications. Here, we show that SpyCatcher proteins, which bind and spontaneously form covalent isopeptide bonds with cognate SpyTag peptides, can conjugate engineered Ab to nanoparticle surfaces with control over stability, orientation, and stoichiometry. Compact SpyCatcher-functionalized QDs and UCNPs may be labeled with short-chain variable fragment Ab (scFv) engineered to bind urokinase-type plasminogen activator receptors (uPAR) that are overexpressed in many human cancers. Confocal imaging of anti-uPAR scFv-QD conjugates shows the antibody mediates specific binding and internalization by breast cancer cells expressing uPAR. Time-lapse imaging of photostable scFv-UCNP conjugates shows that Ab binding causes uPAR internalization with a ∼20 min half-life on the cell surface, and uPAR is internalized to endolysosomal compartments distinct from general membrane stains and without significant recycling to the cell surface. The controlled and stable conjugation of engineered Ab to NPs enables targeting of diverse receptors for live-cell study of their distribution, trafficking, and physiology.
Keywords: antibodies; bioconjugation; cancer cells; quantum dots; receptor trafficking; uPAR; upconverting nanoparticles.
Conflict of interest statement
Competing Financial Interests
The authors declare no competing financial interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

