Synthesis of DNA Duplexes Containing Site-Specific Interstrand Cross-Links via Sequential Reductive Amination Reactions Involving Diamine Linkers and Abasic Sites on Complementary Oligodeoxynucleotides
- PMID: 34694787
- PMCID: PMC8650211
- DOI: 10.1021/acs.chemrestox.1c00293
Synthesis of DNA Duplexes Containing Site-Specific Interstrand Cross-Links via Sequential Reductive Amination Reactions Involving Diamine Linkers and Abasic Sites on Complementary Oligodeoxynucleotides
Abstract
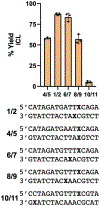
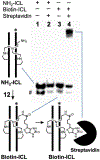
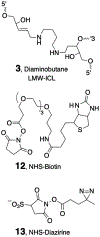
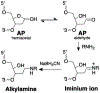
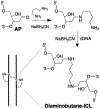
Interstrand DNA cross-links are important in biology, medicinal chemistry, and materials science. Accordingly, methods for the targeted installation of interstrand cross-links in DNA duplexes may be useful in diverse fields. Here, a simple procedure is reported for the preparation of DNA duplexes containing site-specific, chemically defined interstrand cross-links. The approach involves sequential reductive amination reactions between diamine linkers and two abasic (apurinic/apyrimidinic, AP) sites on complementary oligodeoxynucleotides. Use of the symmetrical triamine, tris(2-aminoethyl)amine, in this reaction sequence enabled the preparation of a cross-linked DNA duplex bearing a derivatizable aminoethyl group.
Conflict of interest statement
The authors declare no conflicts of interest
Figures


Similar articles
-
Preparation and Purification of Oligodeoxynucleotide Duplexes Containing a Site-Specific, Reduced, Chemically Stable Covalent Interstrand Cross-Link Between a Guanine Residue and an Abasic Site.Methods Mol Biol. 2019;1973:163-175. doi: 10.1007/978-1-4939-9216-4_10. Methods Mol Biol. 2019. PMID: 31016701 Free PMC article.
-
Interstrand DNA Cross-Links Derived from Reaction of a 2-Aminopurine Residue with an Abasic Site.ACS Chem Biol. 2019 Jul 19;14(7):1481-1489. doi: 10.1021/acschembio.9b00208. Epub 2019 Jul 1. ACS Chem Biol. 2019. PMID: 31259519 Free PMC article.
-
On the formation and properties of interstrand DNA-DNA cross-links forged by reaction of an abasic site with the opposing guanine residue of 5'-CAp sequences in duplex DNA.J Am Chem Soc. 2013 Jan 23;135(3):1015-25. doi: 10.1021/ja308119q. Epub 2013 Jan 11. J Am Chem Soc. 2013. PMID: 23215239 Free PMC article.
-
Synthesis of G-N2-(CH2)3-N2-G Trimethylene DNA Interstrand Cross-Links.Curr Protoc Nucleic Acid Chem. 2014 Mar 26;56:5.14.1-15. doi: 10.1002/0471142700.nc0514s56. Curr Protoc Nucleic Acid Chem. 2014. PMID: 25606979 Review.
-
Preparation of interstrand cross-linked DNA oligonucleotide duplexes.Front Biosci. 2004 Jan 1;9:421-37. doi: 10.2741/1246. Front Biosci. 2004. PMID: 14766379 Review.
Cited by
-
Reconsidering the Chemical Nature of Strand Breaks Derived from Abasic Sites in Cellular DNA: Evidence for 3'-Glutathionylation.J Am Chem Soc. 2022 Jun 15;144(23):10471-10482. doi: 10.1021/jacs.2c02703. Epub 2022 May 25. J Am Chem Soc. 2022. PMID: 35612610 Free PMC article.
References
-
- Imani-Nejad M, Johnson KM, Price NE, and Gates KS (2016) A new cross-link for an old cross-linking drug: the nitrogen mustard anticancer agent mechlorethamine generates cross-links derived from abasic sites in addition to the expected drug-bridged cross-links. Biochemistry 55, 7033–7041. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

