Electrochemical Capillary-Flow Immunoassay for Detecting Anti-SARS-CoV-2 Nucleocapsid Protein Antibodies at the Point of Care
- PMID: 34694794
- PMCID: PMC8565458
- DOI: 10.1021/acssensors.1c01527
Electrochemical Capillary-Flow Immunoassay for Detecting Anti-SARS-CoV-2 Nucleocapsid Protein Antibodies at the Point of Care
Abstract
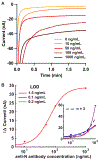
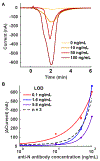
Rapid and inexpensive serological tests for SARS-CoV-2 antibodies are needed to conduct population-level seroprevalence surveillance studies and can improve diagnostic reliability when used in combination with viral tests. Here, we report a novel low-cost electrochemical capillary-flow device to quantify IgG antibodies targeting SARS-CoV-2 nucleocapsid proteins (anti-N antibody) down to 5 ng/mL in low-volume (10 μL) human whole blood samples in under 20 min. No sample preparation is needed as the device integrates a blood-filtration membrane for on-board plasma extraction. The device is made of stacked layers of a hydrophilic polyester and double-sided adhesive films, which create a passive microfluidic circuit that automates the steps of an enzyme-linked immunosorbent assay (ELISA). The sample and reagents are sequentially delivered to a nitrocellulose membrane that is modified with a recombinant SARS-CoV-2 nucleocapsid protein. When present in the sample, anti-N antibodies are captured on the nitrocellulose membrane and detected via chronoamperometry performed on a screen-printed carbon electrode. As a result of this quantitative electrochemical readout, no result interpretation is required, making the device ideal for point-of-care (POC) use by non-trained users. Moreover, we show that the device can be coupled to a near-field communication potentiostat operated from a smartphone, confirming its true POC potential. The novelty of this work resides in the integration of sensitive electrochemical detection with capillary-flow immunoassay, providing accuracy at the point of care. This novel electrochemical capillary-flow device has the potential to aid the diagnosis of infectious diseases at the point of care.
Keywords: SARS-CoV-2; capillary-flow device; electrochemistry; point-of-care (POC) diagnostics; serology.
Figures
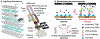


References
-
- WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus Disease (COVID-19) Dashboard https://covid19.who.int/ (accessed Jul 10, 2021).
-
- Testing, Screening, and Outbreak Response for Institutions of Higher Education (IHEs) | CDC; https://www.cdc.gov/coronavirus/2019-ncov/community/colleges-universitie... (accessed Mar 12, 2021).
-
- Pollán M; Pérez-Gómez B; Pastor-Barriuso R; Oteo J; Hernán MA; Pérez-Olmeda M; Sanmartín JL; Fernández-García A; Cruz I; Fernández de Larrea N; et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): A Nationwide, Population-Based Seroepidemiological Study. Lancet 2020, 396 (10250), 535–544. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

