Synergy Mechanisms of Daptomycin-Fosfomycin Combinations in Daptomycin-Susceptible and -Resistant Methicillin-Resistant Staphylococcus aureus: In Vitro, Ex Vivo, and In Vivo Metrics
- PMID: 34694870
- PMCID: PMC8765261
- DOI: 10.1128/AAC.01649-21
Synergy Mechanisms of Daptomycin-Fosfomycin Combinations in Daptomycin-Susceptible and -Resistant Methicillin-Resistant Staphylococcus aureus: In Vitro, Ex Vivo, and In Vivo Metrics
Abstract
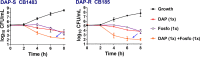
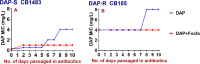
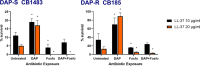
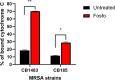
Increased usage of daptomycin (DAP) for methicillin-resistant Staphylococcus aureus (MRSA) infections has led to emergence of DAP-resistant (DAP-R) strains, resulting in treatment failures. DAP-fosfomycin (Fosfo) combinations are synergistically active against MRSA, although the mechanism(s) of this interaction is not fully understood. The current study explored four unique but likely interrelated activities of DAP-Fosfo combinations: (i) synergistic killing, (ii) prevention of evolution of DAP-R, (iii) resensitization of already DAP-R subpopulations to a DAP-susceptible (DAP-S) phenotype, and (iv) perturbations of specific cell envelope phenotypes known to correlate with DAP-R in MRSA. Using an isogenic DAP-S (CB1483)/DAP-R (CB185) clinical MRSA strain pair, we demonstrated that combinations of DAP plus Fosfo (DAP+Fosfo) (i) enhanced killing of both strains in vitro and ex vivo, (ii) increased target tissue clearances of the DAP-R strain in an in vivo model of experimental infective endocarditis (IE), (iii) prevented emergence of DAP-R in the DAP-S parental strain both in vitro and ex vivo, and (iv) resensitized the DAP-R strain to a DAP-S phenotype ex vivo. Phenotypically, following exposure to sub-MIC Fosfo, the DAP-S/DAP-R strain pair exhibited distinct modifications in (i) net positive surface charge (P < 0.05), (ii) quantity (P < 0.0001) and localization of cell membrane cardiolipin (CL), (iii) DAP surface binding, and (iv) membrane fluidity (P < 0.05). Furthermore, preconditioning this strain pair to DAP with or without Fosfo (DAP+/-Fosfo) sensitized these organisms to killing by the human host defense peptide LL37. These data underscore the notion that DAP-Fosfo combinations can impact MRSA clearances within multiple microenvironments, likely based on specific phenotypic adaptations.
Keywords: MRSA; Staphylococcus; combination; daptomycin; fosfomycin.
Figures


Similar articles
-
Increased cell wall teichoic acid production and D-alanylation are common phenotypes among daptomycin-resistant methicillin-resistant Staphylococcus aureus (MRSA) clinical isolates.PLoS One. 2013 Jun 13;8(6):e67398. doi: 10.1371/journal.pone.0067398. Print 2013. PLoS One. 2013. PMID: 23785522 Free PMC article.
-
β-Lactam-Induced Cell Envelope Adaptations, Not Solely Enhanced Daptomycin Binding, Underlie Daptomycin-β-Lactam Synergy in Methicillin-Resistant Staphylococcus aureus.Antimicrob Agents Chemother. 2021 Jul 16;65(8):e0035621. doi: 10.1128/AAC.00356-21. Epub 2021 Jul 16. Antimicrob Agents Chemother. 2021. PMID: 34097478 Free PMC article.
-
Phenotypic and genotypic correlates of daptomycin-resistant methicillin-susceptible Staphylococcus aureus clinical isolates.J Microbiol. 2017 Feb;55(2):153-159. doi: 10.1007/s12275-017-6509-1. Epub 2017 Jan 26. J Microbiol. 2017. PMID: 28120188 Free PMC article.
-
Fosfomycin as salvage therapy for persistent methicillin-resistant Staphylococcus aureus bacteremia: A case series and review of the literature.J Infect Chemother. 2024 Apr;30(4):352-356. doi: 10.1016/j.jiac.2023.10.024. Epub 2023 Nov 3. J Infect Chemother. 2024. PMID: 37922987 Review.
-
Vancomycin or Daptomycin Plus a β-Lactam Versus Vancomycin or Daptomycin Alone for Methicillin-Resistant Staphylococcus aureus Bloodstream Infections: A Systematic Review and Meta-Analysis.Microb Drug Resist. 2021 Aug;27(8):1044-1056. doi: 10.1089/mdr.2020.0350. Epub 2021 Mar 15. Microb Drug Resist. 2021. PMID: 33728980
Cited by
-
Synergistic antibacterial activity and prevention of drug resistance of daptomycin combined with fosfomycin against methicillin-resistant Staphylococcus aureus.Antimicrob Agents Chemother. 2025 Aug 6;69(8):e0160924. doi: 10.1128/aac.01609-24. Epub 2025 Jun 18. Antimicrob Agents Chemother. 2025. PMID: 40530996 Free PMC article.
-
Repeated Emergence of Variant TetR Family Regulator, FarR, and Increased Resistance to Antimicrobial Unsaturated Fatty Acid among Clonal Complex 5 Methicillin-Resistant Staphylococcus aureus.Antimicrob Agents Chemother. 2023 Mar 16;67(3):e0074922. doi: 10.1128/aac.00749-22. Epub 2023 Feb 6. Antimicrob Agents Chemother. 2023. PMID: 36744906 Free PMC article.
-
New Antimicrobials and New Therapy Strategies for Endocarditis: Weapons That Should Be Defended.J Clin Med. 2023 Dec 14;12(24):7693. doi: 10.3390/jcm12247693. J Clin Med. 2023. PMID: 38137762 Free PMC article. Review.
-
Genetic Correlates of Synergy Mechanisms of Daptomycin Plus Fosfomycin in Daptomycin-Susceptible and -Resistant Methicillin-Resistant Staphylococcus aureus (MRSA).Microorganisms. 2025 Jun 30;13(7):1532. doi: 10.3390/microorganisms13071532. Microorganisms. 2025. PMID: 40732039 Free PMC article.
-
Targeted Therapy of Severe Infections Caused by Staphylococcus aureus in Critically Ill Adult Patients: A Multidisciplinary Proposal of Therapeutic Algorithms Based on Real-World Evidence.Microorganisms. 2023 Feb 3;11(2):394. doi: 10.3390/microorganisms11020394. Microorganisms. 2023. PMID: 36838359 Free PMC article.
References
-
- Marty FM, Yeh WW, Wennersten CB, Venkataraman L, Albano E, Alyea EP, Gold HS, Baden LR, Pillai SK. 2006. Emergence of a clinical daptomycin-resistant Staphylococcus aureus isolate during treatment of methicillin-resistant Staphylococcus aureus bacteremia and osteomyelitis. J Clin Microbiol 44:595–597. 10.1128/JCM.44.2.595-597.2006. - DOI - PMC - PubMed
-
- Pujol M, Miró J-M, Shaw E, Aguado J-M, San-Juan R, Puig-Asensio M, Pigrau C, Calbo E, Montejo M, Rodriguez-Álvarez R, Garcia-Pais M-J, Pintado V, Escudero-Sánchez R, Lopez-Contreras J, Morata L, Montero M, Andrés M, Pasquau J, Arenas M-D-M, Padilla B, Murillas J, Jover-Sáenz A, López-Cortes L-E, García-Pardo G, Gasch O, Videla S, Hereu P, Tebé C, Pallarès N, Sanllorente M, Domínguez M-Á, Càmara J, Ferrer A, Padullés A, Cuervo G, Carratalà J, MRSA Bacteremia (BACSARM) Trial Investigators. 2021. Daptomycin plus fosfomycin versus daptomycin alone for methicillin-resistant Staphylococcus aureus bacteremia and endocarditis: a randomized clinical trial. Clin Infect Dis 72:1517–1525. 10.1093/cid/ciaa1081. - DOI - PMC - PubMed

