Integrase Inhibitor Resistance Mechanisms and Structural Characteristics in Antiretroviral Therapy-Experienced, Integrase Inhibitor-Naive Adults with HIV-1 Infection Treated with Dolutegravir plus Two Nucleoside Reverse Transcriptase Inhibitors in the DAWNING Study
- PMID: 34694877
- PMCID: PMC8765460
- DOI: 10.1128/AAC.01643-21
Integrase Inhibitor Resistance Mechanisms and Structural Characteristics in Antiretroviral Therapy-Experienced, Integrase Inhibitor-Naive Adults with HIV-1 Infection Treated with Dolutegravir plus Two Nucleoside Reverse Transcriptase Inhibitors in the DAWNING Study
Erratum in
-
Correction for Underwood et al., "Integrase Inhibitor Resistance Mechanisms and Structural Characteristics in Antiretroviral Therapy-Experienced, Integrase Inhibitor-Naive Adults with HIV-1 Infection Treated with Dolutegravir plus Two Nucleoside Reverse Transcriptase Inhibitors in the DAWNING Study".Antimicrob Agents Chemother. 2023 Mar 16;67(3):e0032622. doi: 10.1128/aac.00326-22. Epub 2023 Feb 16. Antimicrob Agents Chemother. 2023. PMID: 36794938 Free PMC article. No abstract available.
Abstract
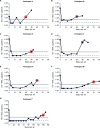
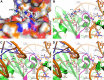
At week 48 in the phase IIIb DAWNING study, the integrase strand transfer inhibitor (INSTI) dolutegravir plus 2 nucleoside reverse transcriptase inhibitors demonstrated superiority to ritonavir-boosted lopinavir in achieving virologic suppression in adults with HIV-1 who failed first-line therapy. Here, we report emergent HIV-1 drug resistance and mechanistic underpinnings among dolutegravir-treated adults in DAWNING. Population viral genotyping, phenotyping, and clonal analyses were performed on participants meeting confirmed virologic withdrawal (CVW) criteria on dolutegravir-containing regimens. Dolutegravir binding to and structural changes in HIV-1 integrase-DNA complexes with INSTI resistance-associated substitutions were evaluated. Of participants who received dolutegravir through week 48 plus an additional 110 weeks for this assessment, 6 met CVW criteria with treatment-emergent INSTI resistance-associated substitutions and 1 had R263R/K at baseline but not at CVW. All 7 achieved HIV-1 RNA levels of <400 copies/mL (5 achieved <50 copies/mL) before CVW. Treatment-emergent G118R was detected in 5 participants, occurring with ≥2 other integrase substitutions, including R263R/K, in 3 participants and without other integrase substitutions in 2 participants. G118R or R263K increased the rate of dolutegravir dissociation from integrase-DNA complexes versus wild-type but retained prolonged binding. Overall, among treatment-experienced adults who received dolutegravir in DAWNING, 6 of 314 participants developed treatment-emergent INSTI resistance-associated substitutions, with a change in in vitro dolutegravir resistance of >10-fold and reduced viral replication capacity versus baseline levels. This study demonstrates that the pathway to dolutegravir resistance is a challenging balance between HIV-1 phenotypic change and associated loss of viral fitness. (This study has been registered at ClinicalTrials.gov under identifier NCT02227238.).
Keywords: HIV-1 infection; antiretroviral agents; barrier to resistance; dolutegravir; integrase strand transfer inhibitor.
Figures


Similar articles
-
Emergence of Resistance in HIV-1 Integrase with Dolutegravir Treatment in a Pediatric Population from the IMPAACT P1093 Study.Antimicrob Agents Chemother. 2022 Jan 18;66(1):e0164521. doi: 10.1128/AAC.01645-21. Epub 2021 Oct 25. Antimicrob Agents Chemother. 2022. PMID: 34694878 Free PMC article. Clinical Trial.
-
Short Communication: Dolutegravir-Based Regimens Are Active in Integrase Strand Transfer Inhibitor-Naive Patients with Nucleoside Reverse Transcriptase Inhibitor Resistance.AIDS Res Hum Retroviruses. 2018 Apr;34(4):343-346. doi: 10.1089/AID.2017.0184. Epub 2018 Mar 22. AIDS Res Hum Retroviruses. 2018. PMID: 29444582 Free PMC article.
-
The Combination of the R263K and T66I Resistance Substitutions in HIV-1 Integrase Is Incompatible with High-Level Viral Replication and the Development of High-Level Drug Resistance.J Virol. 2015 Nov;89(22):11269-74. doi: 10.1128/JVI.01881-15. Epub 2015 Aug 26. J Virol. 2015. PMID: 26311878 Free PMC article.
-
Dolutegravir, a second-generation integrase inhibitor for the treatment of HIV-1 infection.Ann Pharmacother. 2014 Mar;48(3):395-403. doi: 10.1177/1060028013513558. Epub 2013 Nov 19. Ann Pharmacother. 2014. PMID: 24259658 Review.
-
Abacavir/dolutegravir/lamivudine single-tablet regimen: a review of its use in HIV-1 infection.Drugs. 2015 Apr;75(5):503-14. doi: 10.1007/s40265-015-0361-6. Drugs. 2015. PMID: 25698454 Review.
Cited by
-
Longer-term virologic outcomes on tenofovir-lamivudine-dolutegravir in second-line ART.South Afr J HIV Med. 2025 Apr 30;26(1):1677. doi: 10.4102/sajhivmed.v26i1.1677. eCollection 2025. South Afr J HIV Med. 2025. PMID: 40356938 Free PMC article.
-
Synergistic pathogenesis: exploring biofilms, efflux pumps and secretion systems in Acinetobacter baumannii and Staphylococcus aureus.Arch Microbiol. 2025 May 2;207(6):134. doi: 10.1007/s00203-025-04336-w. Arch Microbiol. 2025. PMID: 40314822 Review.
-
Initial Supplementary Dose of Dolutegravir in Second-Line Antiretroviral Therapy: A Noncomparative, Double-Blind, Randomized Placebo-Controlled Trial.Clin Infect Dis. 2023 May 24;76(10):1832-1840. doi: 10.1093/cid/ciad023. Clin Infect Dis. 2023. PMID: 36645792 Free PMC article. Clinical Trial.
-
N-Substituted Bicyclic Carbamoyl Pyridones: Integrase Strand Transfer Inhibitors that Potently Inhibit Drug-Resistant HIV-1 Integrase Mutants.ACS Infect Dis. 2024 Mar 8;10(3):917-927. doi: 10.1021/acsinfecdis.3c00525. Epub 2024 Feb 12. ACS Infect Dis. 2024. PMID: 38346249 Free PMC article.
-
Genotypic correlates of resistance to the HIV-1 strand transfer integrase inhibitor cabotegravir.Antiviral Res. 2022 Dec;208:105427. doi: 10.1016/j.antiviral.2022.105427. Epub 2022 Sep 30. Antiviral Res. 2022. PMID: 36191692 Free PMC article.
References
-
- Cahn P, Pozniak AL, Mingrone H, Shuldyakov A, Brites C, Andrade-Villanueva JF, Richmond G, Beltran Buendia C, Fourie J, Ramgopal M, Hagins D, Felizarta F, Madruga J, Reuter T, Newman T, Small CB, Lombaard J, Grinsztejn B, Dorey D, Underwood M, Griffith S, Min S. 2013. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet 382:700–708. 10.1016/S0140-6736(13)61221-0. - DOI - PubMed
-
- Walmsley SL, Antela A, Clumeck N, Duiculescu D, Eberhard A, Gutiérrez F, Hocqueloux L, Maggiolo F, Sandkovsky U, Granier C, Pappa K, Wynne B, Min S, Nichols G. 2013. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med 369:1807–1818. 10.1056/NEJMoa1215541. - DOI - PubMed
-
- Kobayashi M, Yoshinaga T, Seki T, Wakasa-Morimoto C, Brown KW, Ferris R, Foster SA, Hazen RJ, Miki S, Suyama-Kagitani A, Kawauchi-Miki S, Taishi T, Kawasuji T, Johns BA, Underwood MR, Garvey EP, Sato A, Fujiwara T. 2011. In vitro antiretroviral properties of S/GSK1349572, a next-generation HIV integrase inhibitor. Antimicrob Agents Chemother 55:813–821. 10.1128/AAC.01209-10. - DOI - PMC - PubMed
-
- Raffi F, Rachlis A, Stellbrink H-J, Hardy WD, Torti C, Orkin C, Bloch M, Podzamczer D, Pokrovsky V, Pulido F, Almond S, Margolis D, Brennan C, Min S. 2013. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet 381:735–743. 10.1016/S0140-6736(12)61853-4. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

