Potency and Preclinical Evidence of Synergy of Oral Azole Drugs and Miltefosine in an Ex Vivo Model of Leishmania (Viannia) panamensis Infection
- PMID: 34694879
- PMCID: PMC8765415
- DOI: 10.1128/AAC.01425-21
Potency and Preclinical Evidence of Synergy of Oral Azole Drugs and Miltefosine in an Ex Vivo Model of Leishmania (Viannia) panamensis Infection
Abstract
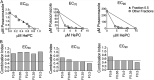
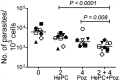
Failure of treatment of cutaneous leishmaniasis with antimonial drugs and miltefosine is frequent. Use of oral combination therapy represents an attractive strategy to increase efficacy of treatment and reduce the risk of drug resistance. We evaluated the potency of posaconazole, itraconazole, voriconazole, and fluconazole and the potential synergy of those demonstrating the highest potency, in combination with miltefosine (HePC), against infection with Leishmania (Viannia) panamensis. Synergistic activity was determined by isobolograms and calculation of the fractional inhibitory concentration index (FICI), based on parasite quantification using an ex vivo model of human peripheral blood mononuclear cells (PBMCs) infected with a luciferase-transfected, antimony and miltefosine sensitive line of L. panamensis. The drug combination and concentrations that displayed synergy were then evaluated for antileishmanial effect in 10 clinical strains of L. panamensis by reverse transcription-quantitative (qRT-PCR) of Leishmania 7SLRNA. High potency was substantiated for posaconazole and itraconazole against sensitive as well as HePC- and antimony-resistant lines of L. panamensis, whereas fluconazole and voriconazole displayed low potency. HePC combined with posaconazole (Poz) demonstrated evidence of synergy at free drug concentrations achieved in plasma during treatment (2 μM HePC plus 4 μM Poz). FICI, based on 70% and 90% reduction of infection, was 0.5 for the sensitive line. The combination of 2 μM HePC plus 4 μM Poz effected a significantly greater reduction of infection by clinical strains of L. panamensis than individual drugs. Orally administrable miltefosine/posaconazole combinations demonstrated synergistic antileishmanial capacity ex vivo against L. panamensis, supporting their potential as a novel therapeutic strategy to improve efficacy and effectiveness of treatment.
Keywords: Leishmania; azoles; combined therapy; miltefosine; posaconazole.
Figures


References
-
- Llanos-Cuentas A, Tulliano G, Araujo-Castillo R, Miranda-Verastegui C, Santamaria-Castrellon G, Ramirez L, Lazo M, De Doncker S, Boelaert M, Robays J, Dujardin J-C, Arevalo J, Chappuis F. 2008. Clinical and parasite species risk factors for pentavalent antimonial treatment failure in cutaneous leishmaniasis in Peru. Clin Infect Dis 46:223–231. doi:10.1086/524042. - DOI - PubMed
-
- Rubiano LC, Miranda MC, Muvdi Arenas S, Montero LM, Rodríguez-Barraquer I, Garcerant D, Prager M, Osorio L, Rojas MX, Pérez M, Nicholls RS, Gore Saravia N. 2012. Noninferiority of miltefosine versus meglumine antimoniate for cutaneous leishmaniasis in children. J Infect Dis 205:684–692. doi:10.1093/infdis/jir816. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

