Effect of Elective Internal Mammary Node Irradiation on Disease-Free Survival in Women With Node-Positive Breast Cancer: A Randomized Phase 3 Clinical Trial
- PMID: 34695841
- PMCID: PMC8546620
- DOI: 10.1001/jamaoncol.2021.6036
Effect of Elective Internal Mammary Node Irradiation on Disease-Free Survival in Women With Node-Positive Breast Cancer: A Randomized Phase 3 Clinical Trial
Abstract
Importance: The benefit of internal mammary node irradiation (IMNI) for treatment outcomes in node-positive breast cancer is unknown.
Objective: To investigate whether the inclusion of IMNI in regional nodal irradiation improves disease-free survival (DFS) in women with node-positive breast cancer.
Design, setting, and participants: This multicenter, phase 3 randomized clinical trial was conducted from June 1, 2008, to February 29, 2020, at 13 hospitals in South Korea. Women with pathologically confirmed, node-positive breast cancer after breast-conservation surgery or mastectomy with axillary lymph node dissection were eligible and enrolled between November 19, 2008, and January 14, 2013. Patients with distant metastasis and those who had received neoadjuvant treatment were excluded. Data analyses were performed according to the intention-to-treat principle.
Interventions: All patients underwent regional nodal irradiation along with breast or chest wall irradiation. They were randomized 1:1 to receive radiotherapy either with IMNI or without IMNI.
Main outcomes and measures: The primary end point was the 7-year DFS. Secondary end points included the rates of overall survival, breast cancer-specific survival, and toxic effects.
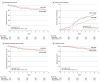
Results: A total of 735 women (mean [SD] age, 49.0 [9.1] years) were included in the analyses, of whom 373 received regional nodal irradiation without IMNI and 362 received regional nodal irradiation with IMNI. Nearly all patients underwent taxane-based adjuvant systemic treatment. The median (IQR) follow-up was 100.4 (89.7-112.1) months. The 7-year DFS rates did not significantly differ between the groups treated without IMNI and with IMNI (81.9% vs 85.3%; hazard ratio [HR], 0.80; 95% CI, 0.57-1.14; log-rank P = .22). However, an ad hoc subgroup analysis showed significantly higher DFS rates with IMNI among patients with mediocentrally located tumors. In this subgroup, the 7-year DFS rates were 81.6% without IMNI vs 91.8% with IMNI (HR, 0.42; 95% CI, 0.22-0.82; log-rank P = .008), and the 7-year breast cancer mortality rates were 10.2% without IMNI vs 4.9% with IMNI (HR, 0.41; 95% CI, 0.17-0.99; log-rank P = .04). No differences were found between the 2 groups in the incidence of adverse effects, including cardiac toxic effects and radiation pneumonitis.
Conclusions and relevance: This randomized clinical trial found that including IMNI in regional nodal irradiation did not significantly improve the DFS in patients with node-positive breast cancer. However, patients with medially or centrally located tumors may benefit from the use of IMNI.
Trial registration: ClinicalTrials.gov Identifier: NCT04803266.
Conflict of interest statement
Figures

Comment in
-
Internal Mammary Nodal Irradiation Debate for Node-Positive Breast Cancer-Has the Needle Moved?-Reply.JAMA Oncol. 2022 May 1;8(5):780-781. doi: 10.1001/jamaoncol.2022.0229. JAMA Oncol. 2022. PMID: 35323845 No abstract available.
-
Internal Mammary Nodal Irradiation Debate for Node-Positive Breast Cancer-Has the Needle Moved?JAMA Oncol. 2022 May 1;8(5):780. doi: 10.1001/jamaoncol.2022.0226. JAMA Oncol. 2022. PMID: 35323866 No abstract available.
-
The Internal Mammary Node Irradiation Debate in Node-Positive Breast Cancer: Case Closed.Int J Radiat Oncol Biol Phys. 2023 Nov 15;117(4):779-782. doi: 10.1016/j.ijrobp.2023.07.021. Int J Radiat Oncol Biol Phys. 2023. PMID: 37838444 No abstract available.
References
-
- McGale P, Taylor C, Correa C, et al. ; EBCTCG (Early Breast Cancer Trialists’ Collaborative Group) . Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127-2135. doi: 10.1016/S0140-6736(14)60488-8 - DOI - PMC - PubMed
-
- Clarke M, Collins R, Darby S, et al. ; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087-2106. doi: 10.1016/S0140-6736(05)67887-7 - DOI - PubMed

