A Review of Non-Invasive Optical Systems for Continuous Blood Glucose Monitoring
- PMID: 34696033
- PMCID: PMC8537963
- DOI: 10.3390/s21206820
A Review of Non-Invasive Optical Systems for Continuous Blood Glucose Monitoring
Abstract
The prevalence of diabetes is increasing globally. More than 690 million cases of diabetes are expected worldwide by 2045. Continuous blood glucose monitoring is essential to control the disease and avoid long-term complications. Diabetics suffer on a daily basis with the traditional glucose monitors currently in use, which are invasive, painful, and cost-intensive. Therefore, the demand for non-invasive, painless, economical, and reliable approaches to monitor glucose levels is increasing. Since the last decades, many glucose sensing technologies have been developed. Researchers and scientists have been working on the enhancement of these technologies to achieve better results. This paper provides an updated review of some of the pioneering non-invasive optical techniques for monitoring blood glucose levels that have been proposed in the last six years, including a summary of state-of-the-art error analysis and validation techniques.
Keywords: Raman; diabetes; fluorescent; glucose; infrared; non-invasive; optics; photoacoustic; spectroscopy; terahertz.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

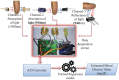
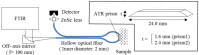


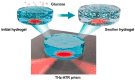
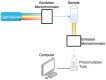

References
-
- Davis B. What Is the Pathophysiology of Diabetes Mellitus? [(accessed on 6 October 2021)]. Available online: https://www.mvorganizing.org/what-is-the-pathophysiology-of-diabetes-mel...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

