Generation of Variability in Chrysodeixis includens Nucleopolyhedrovirus (ChinNPV): The Role of a Single Variant
- PMID: 34696324
- PMCID: PMC8539094
- DOI: 10.3390/v13101895
Generation of Variability in Chrysodeixis includens Nucleopolyhedrovirus (ChinNPV): The Role of a Single Variant
Abstract
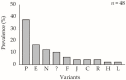
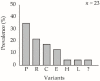
The mechanisms generating variability in viruses are diverse. Variability allows baculoviruses to evolve with their host and with changes in their environment. We examined the role of one genetic variant of Chrysodeixis includens nucleopolyhedrovirus (ChinNPV) and its contribution to the variability of the virus under laboratory conditions. A mixture of natural isolates (ChinNPV-Mex1) contained two genetic variants that dominated over other variants in individual larvae that consumed high (ChinNPV-K) and low (ChinNPV-E) concentrations of inoculum. Studies on the ChinNPV-K variant indicated that it was capable of generating novel variation in a concentration-dependent manner. In cell culture, cells inoculated with high concentrations of ChinNPV-K produced OBs with the ChinNPV-K REN profile, whereas a high diversity of ChinNPV variants was recovered following plaque purification of low concentrations of ChinNPV-K virion inoculum. Interestingly, the ChinNPV-K variant could not be recovered from plaques derived from low concentration inocula originating from budded virions or occlusion-derived virions of ChinNPV-K. Genome sequencing revealed marked differences between ChinNPV-K and ChinNPV-E, with high variation in the ChinNPV-K genome, mostly due to single nucleotide polymorphisms. We conclude that ChinNPV-K is an unstable genetic variant that is responsible for generating much of the detected variability in the natural ChinNPV isolates used in this study.
Keywords: ChinNPV; Chrysodeixis includens; SNPs; concentration; prevalence; variability.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures






Similar articles
-
Genetic Variability of Chrysodeixis Includens Nucleopolyhedrovirus (ChinNPV) and the Insecticidal Characteristics of Selected Genotypic Variants.Viruses. 2019 Jun 26;11(7):581. doi: 10.3390/v11070581. Viruses. 2019. PMID: 31247955 Free PMC article.
-
A Novel Alphabaculovirus from the Soybean Looper, Chrysodeixis includens, that Produces Tetrahedral Occlusion Bodies and Encodes Two Copies of he65.Viruses. 2019 Jun 26;11(7):579. doi: 10.3390/v11070579. Viruses. 2019. PMID: 31247912 Free PMC article.
-
Mosaic genome evolution and phylogenetics of Chrysodeixis includens nucleopolyhedrovirus (ChinNPV) and virulence of seven new isolates from the Brazilian states of Minas Gerais and Mato Grosso.Arch Virol. 2021 Jan;166(1):125-138. doi: 10.1007/s00705-020-04858-2. Epub 2020 Oct 27. Arch Virol. 2021. PMID: 33111162
-
No cross-resistance between ChinNPV and chemical insecticides in Chrysodeixis includens (Lepidoptera: Noctuidae).J Invertebr Pathol. 2019 Jun;164:66-68. doi: 10.1016/j.jip.2019.05.001. Epub 2019 May 9. J Invertebr Pathol. 2019. PMID: 31078547
-
Baseline Susceptibility of Brazilian Populations of Chrysodeixis includens (Lepidoptera: Noctuidae) to C. includens Nucleopolyhedrovirus and Diagnostic Concentration for Resistance Monitoring.J Econ Entomol. 2019 Feb 12;112(1):349-354. doi: 10.1093/jee/toy361. J Econ Entomol. 2019. PMID: 30476204
Cited by
-
Nucleopolyhedrovirus Coocclusion Technology: A New Concept in the Development of Biological Insecticides.Front Microbiol. 2022 Jan 25;12:810026. doi: 10.3389/fmicb.2021.810026. eCollection 2021. Front Microbiol. 2022. PMID: 35145496 Free PMC article. Review.
-
Coocclusion of Helicoverpa armigera Single Nucleopolyhedrovirus (HearSNPV) and Helicoverpa armigera Multiple Nucleopolyhedrovirus (HearMNPV): Pathogenicity and Stability in Homologous and Heterologous Hosts.Viruses. 2022 Mar 26;14(4):687. doi: 10.3390/v14040687. Viruses. 2022. PMID: 35458418 Free PMC article.
-
Empirical estimates of the mutation rate for an alphabaculovirus.PLoS Genet. 2022 Jun 6;18(6):e1009806. doi: 10.1371/journal.pgen.1009806. eCollection 2022 Jun. PLoS Genet. 2022. PMID: 35666722 Free PMC article.
-
Baculovirus Genetic Diversity and Population Structure.Viruses. 2025 Jan 22;17(2):142. doi: 10.3390/v17020142. Viruses. 2025. PMID: 40006898 Free PMC article. Review.
References
-
- Harrison R.L., Herniou E.A., Jehle J.A., Theilmann D.A., Burand J.P., Becnel J.J., Krell P.J., van Oers M.M., Mowery J.D., Bauchan G.R., et al. ICTV Virus Taxonomy Profile: Baculoviridae. J. Gen. Virol. 2018;99:1185–1186. - PubMed
-
- Erlandson M. Genetic variation in field populations of baculoviruses: Mechanisms for generating variation and its potential role in baculovirus epizootiology. Virol. Sin. 2009;24:458–469.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

