Turmeric Root and Its Bioactive Ingredient Curcumin Effectively Neutralize SARS-CoV-2 In Vitro
- PMID: 34696344
- PMCID: PMC8537626
- DOI: 10.3390/v13101914
Turmeric Root and Its Bioactive Ingredient Curcumin Effectively Neutralize SARS-CoV-2 In Vitro
Abstract
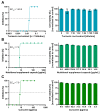
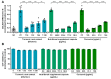
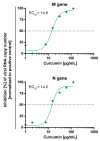
Severe Acute Respiratory Syndrome Coronavirus Type 2 (SARS-CoV-2) is the causative agent of the coronavirus disease 2019 (COVID-19). The availability of effective and well-tolerated antiviral drugs for the treatment of COVID-19 patients is still very limited. Traditional herbal medicines elicit antiviral activity against various viruses and might therefore represent a promising option for the complementary treatment of COVID-19 patients. The application of turmeric root in herbal medicine has a very long history. Its bioactive ingredient curcumin shows a broad-spectrum antimicrobial activity. In the present study, we investigated the antiviral activity of aqueous turmeric root extract, the dissolved content of a curcumin-containing nutritional supplement capsule, and pure curcumin against SARS-CoV-2. Turmeric root extract, dissolved turmeric capsule content, and pure curcumin effectively neutralized SARS-CoV-2 at subtoxic concentrations in Vero E6 and human Calu-3 cells. Furthermore, curcumin treatment significantly reduced SARS-CoV-2 RNA levels in cell culture supernatants. Our data uncover curcumin as a promising compound for complementary COVID-19 treatment. Curcumin concentrations contained in turmeric root or capsules used as nutritional supplements completely neutralized SARS-CoV-2 in vitro. Our data argue in favor of appropriate and carefully monitored clinical studies that vigorously test the effectiveness of complementary treatment of COVID-19 patients with curcumin-containing products.
Keywords: COVID-19; Curcuma longa; SARS-CoV-2; antiviral; curcumin; herbal medicine; turmeric root.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

