Primary HSV-2 Infection in Early Pregnancy Results in Transplacental Viral Transmission and Dose-Dependent Adverse Pregnancy Outcomes in a Novel Mouse Model
- PMID: 34696359
- PMCID: PMC8538385
- DOI: 10.3390/v13101929
Primary HSV-2 Infection in Early Pregnancy Results in Transplacental Viral Transmission and Dose-Dependent Adverse Pregnancy Outcomes in a Novel Mouse Model
Abstract
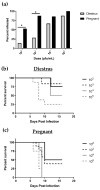
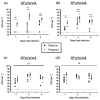
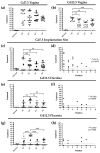
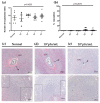
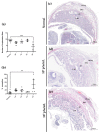
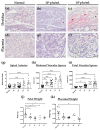
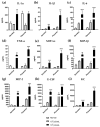
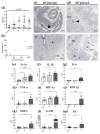
Herpes simplex virus type 2 (HSV-2) infection affects 24 million births annually and is associated with adverse pregnancy outcomes, including neonatal herpes; however, the mechanisms underlying in utero transmission of HSV-2 are largely unknown. We examined the effects of primary HSV-2 infection during early pregnancy on gestational outcomes in a novel, clinically relevant mouse model. Pregnant C57BL/6 mice were infected intravaginally with 102-105 pfu/mL HSV-2 on gestation day (gd) 4.5. Controls were infected, nonpregnant, diestrus-staged mice and pregnant, uninfected mice. Compared to nonpregnant mice, pregnant mice were 100-fold more susceptible to HSV-2 infection. Three days post-inoculation (gd7.5), viral DNA was present in implantation sites, but pregnancy outcomes were largely unaffected by infection. Eight days post-inoculation (gd12.5), HSV-2 DNA persisted in placental tissues, resulting in inflammation and hemorrhage. Fetal and placental weights were reduced and fetal loss was observed with high viral doses. HSV-2 DNA and increased expression of pro-inflammatory mediators were detected in fetal tissues at gd12.5, signifying viral transmission and fetal infection, even with low viral doses. This mouse model shows a dose-dependent effect of primary HSV-2 infection on pregnancy outcomes and suggests that fetal loss may occur due to placental inflammation, thus providing valuable insight into in utero transmission of HSV-2.
Keywords: HSV-2; mouse model; neonatal herpes; placental pathology; pregnancy; viral infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Prevalence of herpes simplex virus types 1 and 2 at maternal and fetal sides of the placenta in asymptomatic pregnant women.Am J Reprod Immunol. 2017 Jul;78(1). doi: 10.1111/aji.12689. Epub 2017 Apr 25. Am J Reprod Immunol. 2017. PMID: 28440579
-
Estradiol regulates susceptibility following primary exposure to genital herpes simplex virus type 2, while progesterone induces inflammation.J Virol. 2005 Mar;79(5):3107-16. doi: 10.1128/JVI.79.5.3107-3116.2005. J Virol. 2005. PMID: 15709030 Free PMC article.
-
[Intrauterine herpes simplex virus infection].Klin Padiatr. 2001 Mar-Apr;213(2):63-8. doi: 10.1055/s-2001-12878. Klin Padiatr. 2001. PMID: 11305194 German.
-
[Herpes simplex virus infection in pregnancy and transmission to neonatal].Akush Ginekol (Sofiia). 2005;44(6):31-5. Akush Ginekol (Sofiia). 2005. PMID: 18982828 Review. Bulgarian.
-
Management of neonatal herpes simplex virus infection.Paediatr Drugs. 2001;3(2):81-90. doi: 10.2165/00128072-200103020-00001. Paediatr Drugs. 2001. PMID: 11269641 Review.
Cited by
-
Roles of TGF-β1 in Viral Infection during Pregnancy: Research Update and Perspectives.Int J Mol Sci. 2023 Mar 30;24(7):6489. doi: 10.3390/ijms24076489. Int J Mol Sci. 2023. PMID: 37047462 Free PMC article. Review.
-
Innate immune responses to pathogens at the maternal-fetal interface.Nat Rev Immunol. 2025 Jun 18. doi: 10.1038/s41577-025-01191-0. Online ahead of print. Nat Rev Immunol. 2025. PMID: 40533582 Review.
-
Pathogenesis of viral infections during pregnancy.Clin Microbiol Rev. 2024 Jun 13;37(2):e0007323. doi: 10.1128/cmr.00073-23. Epub 2024 Feb 29. Clin Microbiol Rev. 2024. PMID: 38421182 Free PMC article. Review.
-
Analysis of HSV1/2 Infection Reveals an Association between HSV-2 Reactivation and Pregnancy.Viruses. 2024 Aug 28;16(9):1370. doi: 10.3390/v16091370. Viruses. 2024. PMID: 39339846 Free PMC article.
-
The role of viral infection in implantation failure: direct and indirect effects.Reprod Biol Endocrinol. 2024 Nov 11;22(1):142. doi: 10.1186/s12958-024-01303-w. Reprod Biol Endocrinol. 2024. PMID: 39529140 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

