Norovirus Epidemiology and Genetic Diversity in Leipzig, Germany during 2013-2017
- PMID: 34696390
- PMCID: PMC8541062
- DOI: 10.3390/v13101961
Norovirus Epidemiology and Genetic Diversity in Leipzig, Germany during 2013-2017
Abstract
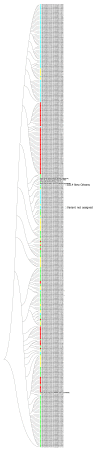
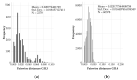
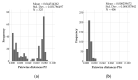
Globally and in all age groups, noroviruses are a main cause of gastroenteritis. To assess their local epidemiology and genetic diversity, stool samples of 7509 inpatients with gastrointestinal complaints from all age groups were analyzed. After detection of norovirus genogroup I and II RNA by real-time RT-PCR, viral capsids were genotyped by partial nucleic acid sequencing. In the case of GII.2 strains, polymerase genotypes were also assessed. Between October 2013 and September 2017, presence of norovirus RNA was shown in 611 samples (8.1%), of which 610 (99.8%) were typed successfully. Norovirus positivity rate was higher in patients aged below five years (14.8%) than in older patients (5.7%). Among the 611 norovirus positive samples, GII.4 (56.6%) strains prevailed, followed by GII.6 (11.3%), GII.3 (11.0%) and GII.2 (9.5%). The most common genogroup I (GGI) genotype was GI.3 (3.6%). In addition, rare genotypes such as GII.13, GII.14 and GII.26 were detected. Interestingly, GII.3 infections were most common in children under the age of five years. Assessment of polymerase genotypes in GII.2 viruses showed a shift from P2 to P16, with higher diversity in P2 sequences. The varying distribution of norovirus genotypes depending on season, age and setting of infection highlights the importance of frequent genotyping as a basis for vaccine development and needful adjustments.
Keywords: anti-norovirus vaccines; diarrhea; genotyping; molecular epidemiology; viral diversity; viral gastroenteritis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Koo H.L., Neill F.H., Estes M.K., Munoz F.M., Cameron A., DuPont H.L., Atmar R.L. Noroviruses: The Most Common Pediatric Viral Enteric Pathogen at a Large University Hospital After Introduction of Rotavirus Vaccination. J. Pediatr. Infect. Dis. Soc. 2013;2:57–60. doi: 10.1093/jpids/pis070. - DOI - PMC - PubMed
-
- Hemming M., Räsänen S., Huhti L., Paloniemi M., Salminen M., Vesikari T. Major Reduction of Rotavirus, but Not Norovirus, Gastroenteritis in Children Seen in Hospital after the Introduction of RotaTeq Vaccine into the National Immunization Programme in Finland. Eur. J. Pediatr. 2013;172:739–746. doi: 10.1007/s00431-013-1945-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

