Dengue Virus Infection: A Tale of Viral Exploitations and Host Responses
- PMID: 34696397
- PMCID: PMC8541669
- DOI: 10.3390/v13101967
Dengue Virus Infection: A Tale of Viral Exploitations and Host Responses
Abstract
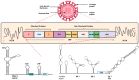
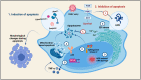
Dengue is a mosquito-borne viral disease (arboviral) caused by the Dengue virus. It is one of the prominent public health problems in tropical and subtropical regions with no effective vaccines. Every year around 400 million people get infected by the Dengue virus, with a mortality rate of about 20% among the patients with severe dengue. The Dengue virus belongs to the Flaviviridae family, and it is an enveloped virus with positive-sense single-stranded RNA as the genetic material. Studies of the infection cycle of this virus revealed potential host targets important for the virus replication cycle. Here in this review article, we will be discussing different stages of the Dengue virus infection cycle inside mammalian host cells and how host proteins are exploited by the virus in the course of infection as well as how the host counteracts the virus by eliciting different antiviral responses.
Keywords: Antibody Dependent Enhancement (ADE); Dengue genome; Dengue virus; apoptosis; autophagy; cytokine storm; host immune response; inflammation; viral pathogenesis; viral replication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- WHO . Dengue and Severe Dengue. WHO; Geneva, Switzerland: 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

