Cell Fusion and Syncytium Formation in Betaherpesvirus Infection
- PMID: 34696402
- PMCID: PMC8537622
- DOI: 10.3390/v13101973
Cell Fusion and Syncytium Formation in Betaherpesvirus Infection
Abstract
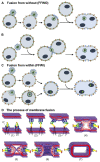
Cell-cell fusion is a fundamental and complex process that occurs during reproduction, organ and tissue growth, cancer metastasis, immune response, and infection. All enveloped viruses express one or more proteins that drive the fusion of the viral envelope with cellular membranes. The same proteins can mediate the fusion of the plasma membranes of adjacent cells, leading to the formation of multinucleated syncytia. While cell-cell fusion triggered by alpha- and gammaherpesviruses is well-studied, much less is known about the fusogenic potential of betaherpesviruses such as human cytomegalovirus (HCMV) and human herpesviruses 6 and 7 (HHV-6 and HHV-7). These are slow-growing viruses that are highly prevalent in the human population and associated with several diseases, particularly in individuals with an immature or impaired immune system such as fetuses and transplant recipients. While HHV-6 and HHV-7 are strictly lymphotropic, HCMV infects a very broad range of cell types including epithelial, endothelial, mesenchymal, and myeloid cells. Syncytia have been observed occasionally for all three betaherpesviruses, both during in vitro and in vivo infection. Since cell-cell fusion may allow efficient spread to neighboring cells without exposure to neutralizing antibodies and other host immune factors, viral-induced syncytia may be important for viral dissemination, long-term persistence, and pathogenicity. In this review, we provide an overview of the viral and cellular factors and mechanisms identified so far in the process of cell-cell fusion induced by betaherpesviruses and discuss the possible consequences for cellular dysfunction and pathogenesis.
Keywords: Herpesviridae; cell–cell fusion; cytomegalovirus; envelope glycoproteins; glycoprotein B; glycoprotein H; glycoprotein L; herpesvirus; polykaryocyte; syncytium formation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

