Function Is More Reliable than Quantity to Follow Up the Humoral Response to the Receptor-Binding Domain of SARS-CoV-2-Spike Protein after Natural Infection or COVID-19 Vaccination
- PMID: 34696403
- PMCID: PMC8538099
- DOI: 10.3390/v13101972
Function Is More Reliable than Quantity to Follow Up the Humoral Response to the Receptor-Binding Domain of SARS-CoV-2-Spike Protein after Natural Infection or COVID-19 Vaccination
Abstract
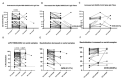
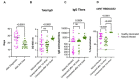
Both the SARS-CoV-2 pandemic and emergence of variants of concern have highlighted the need for functional antibody assays to monitor the humoral response over time. Antibodies directed against the spike (S) protein of SARS-CoV-2 are an important component of the neutralizing antibody response. In this work, we report that in a subset of patients-despite a decline in total S-specific antibodies-neutralizing antibody titers remain at a similar level for an average of 98 days in longitudinal sampling of a cohort of 59 Hispanic/Latino patients exposed to SARS-CoV-2. Our data suggest that 100% of seroconverting patients make detectable neutralizing antibody responses which can be quantified by a surrogate viral neutralization test. Examination of sera from ten out of the 59 subjects which received mRNA-based vaccination revealed that both IgG titers and neutralizing activity of sera were higher after vaccination compared to a cohort of 21 SARS-CoV-2 naïve subjects. One dose was sufficient for the induction of a neutralizing antibody, but two doses were necessary to reach 100% surrogate virus neutralization in subjects irrespective of previous SARS-CoV-2 natural infection status. Like the pattern observed after natural infection, the total anti-S antibodies titers declined after the second vaccine dose; however, neutralizing activity remained relatively constant for more than 80 days after the first vaccine dose. Furthermore, our data indicates that-compared with mRNA vaccination-natural infection induces a more robust humoral immune response in unexposed subjects. This work is an important contribution to understanding the natural immune response to the novel coronavirus in a population severely impacted by SARS-CoV-2. Furthermore, by comparing the dynamics of the immune response after the natural infection vs. the vaccination, these findings suggest that functional neutralizing antibody tests are more relevant indicators than the presence or absence of binding antibodies.
Keywords: COVID-19 vaccine; SARS-CoV-2; neutralization; protection; serology.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Update of
-
Function is more reliable than quantity to follow up the humoral response to the Receptor Binding Domain of SARS- CoV-2 Spike protein after natural infection or COVID-19 vaccination.medRxiv [Preprint]. 2021 Aug 10:2021.06.02.21257975. doi: 10.1101/2021.06.02.21257975. medRxiv. 2021. Update in: Viruses. 2021 Sep 30;13(10):1972. doi: 10.3390/v13101972. PMID: 34100029 Free PMC article. Updated. Preprint.
References
-
- Geers D., Shamier M.C., Bogers S., den Hartog G., Gommers L., Nieuwkoop N.N., Schmitz K.S., Rijsbergen L.C., van Osch J.A.T., Dijkhuizen E., et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci. Immunol. 2021;6:eabj1750. doi: 10.1126/sciimmunol.abj1750. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

