The Value of Rapid Antigen Tests for Identifying Carriers of Viable SARS-CoV-2
- PMID: 34696442
- PMCID: PMC8537476
- DOI: 10.3390/v13102012
The Value of Rapid Antigen Tests for Identifying Carriers of Viable SARS-CoV-2
Abstract
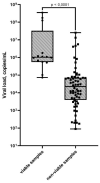
The search for effective methods to detect patients who excrete a viable virus is one of the urgent tasks of modern biomedicine. In the present study, we examined the diagnostic value of two antigen tests, BIOCREDIT COVID-19 Ag (RapiGEN Inc., Anyang, Korea) and SGTI-flex COVID-19 Ag (Sugentech Inc., Cheongju, Korea), for their diagnostic value in identifying patients who excrete viable SARS-CoV-2. As part of the study, we examined samples from 106 patients who had just been admitted to the hospital and who had undergone quantitative RT-PCR and assessment of viability of SARS-CoV-2 using cell culture. Assessment of the tests' value for detecting samples containing viable virus showed high sensitivity for both tests. Sensitivity was 78.6% (95% CI, from 49.2% to 95.3%) for SGTI-flex COVID-19 Ag and 100% (95% CI, from 76.8% to 100%) for Biocredit COVID-19 Ag. The specificity of rapid tests was significantly higher than that of RT-PCR and was 66.3% (95% CI, from 55.7% to 75.8%) and 67.4% (95% CI, from 56.8% to 76.8%) for SGTI-flex COVID-19 Ag and Biocredit COVID-19 Ag versus 30.4% (95% CI, from 21.3% to 40.9%) obtained for PCR. Thus, for tasks of identifying viable SARS-CoV-2 during screening of conditionally healthy people, as well as monitoring those quarantined, rapid tests show significantly better results.
Keywords: COVID-19; SARS-CoV-2; rapid antigen tests; viable virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Imam N., Zaidi S., Chakraborty A.R. Transmission, Prevention, and Risk Factors of COVID-19. In: Kriz C., Imam N., Zaidi S., editors. BREAKING DOWN COVID-19: A Living Textbook. First Medicine and Global Clinical Partners; Miami, FL, USA: 2020. pp. 25–36.
-
- Jungnick S., Hobmaier B., Mautner L., Hoyos M., Haase M., Baiker A., Lahne H., Eberle U., Wimmer C., Hepner S., et al. Detection of the new SARS-CoV-2 variants of concern B.1.1.7 and B.1.351 in five SARS-CoV-2 rapid antigen tests (RATs), Germany, March 2021. Eurosurveillance. 2021;26:2100413. doi: 10.2807/1560-7917.ES.2021.26.16.2100413. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous