USP38 Inhibits Zika Virus Infection by Removing Envelope Protein Ubiquitination
- PMID: 34696459
- PMCID: PMC8538320
- DOI: 10.3390/v13102029
USP38 Inhibits Zika Virus Infection by Removing Envelope Protein Ubiquitination
Abstract
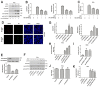
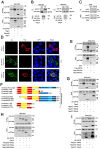
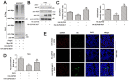
Zika virus (ZIKV) is a mosquito-borne flavivirus, and its infection may cause severe neurodegenerative diseases. The outbreak of ZIKV in 2015 in South America has caused severe human congenital and neurologic disorders. Thus, it is vitally important to determine the inner mechanism of ZIKV infection. Here, our data suggested that the ubiquitin-specific peptidase 38 (USP38) played an important role in host resistance to ZIKV infection, during which ZIKV infection did not affect USP38 expression. Mechanistically, USP38 bound to the ZIKV envelope (E) protein through its C-terminal domain and attenuated its K48-linked and K63-linked polyubiquitination, thereby repressed the infection of ZIKV. In addition, we found that the deubiquitinase activity of USP38 was essential to inhibit ZIKV infection, and the mutant that lacked the deubiquitinase activity of USP38 lost the ability to inhibit infection. In conclusion, we found a novel host protein USP38 against ZIKV infection, and this may represent a potential therapeutic target for the treatment and prevention of ZIKV infection.
Keywords: USP38; Zika virus; deubiquitinase; envelope protein; virus infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Envelope protein ubiquitination drives entry and pathogenesis of Zika virus.Nature. 2020 Sep;585(7825):414-419. doi: 10.1038/s41586-020-2457-8. Epub 2020 Jul 8. Nature. 2020. PMID: 32641828 Free PMC article.
-
Envelope Protein Glycosylation Mediates Zika Virus Pathogenesis.J Virol. 2019 May 29;93(12):e00113-19. doi: 10.1128/JVI.00113-19. Print 2019 Jun 15. J Virol. 2019. PMID: 30944176 Free PMC article.
-
LAMR1 restricts Zika virus infection by attenuating the envelope protein ubiquitination.Virulence. 2021 Dec;12(1):1795-1807. doi: 10.1080/21505594.2021.1948261. Virulence. 2021. PMID: 34282707 Free PMC article.
-
Envelope Protein-Targeting Zika Virus Entry Inhibitors.Int J Mol Sci. 2024 Aug 30;25(17):9424. doi: 10.3390/ijms25179424. Int J Mol Sci. 2024. PMID: 39273370 Free PMC article. Review.
-
Autophagy in Zika Virus Infection: A Possible Therapeutic Target to Counteract Viral Replication.Int J Mol Sci. 2019 Feb 28;20(5):1048. doi: 10.3390/ijms20051048. Int J Mol Sci. 2019. PMID: 30823365 Free PMC article. Review.
Cited by
-
In Memory of the Virologist Jianguo Wu, 1957-2022.Viruses. 2023 Aug 17;15(8):1754. doi: 10.3390/v15081754. Viruses. 2023. PMID: 37632095 Free PMC article.
-
Balancing acts: The posttranslational modification tightrope of flavivirus replication.PLoS Pathog. 2024 Oct 28;20(10):e1012626. doi: 10.1371/journal.ppat.1012626. eCollection 2024 Oct. PLoS Pathog. 2024. PMID: 39466723 Free PMC article. Review.
-
Ubiquitin-specific protease 38 promotes inflammatory atrial fibrillation induced by pressure overload.Europace. 2023 Dec 28;26(1):euad366. doi: 10.1093/europace/euad366. Europace. 2023. PMID: 38288617 Free PMC article.
-
USP38 functions as an oncoprotein by downregulating the p53 pathway through deubiquitination and stabilization of MDM2.Cell Death Differ. 2025 Jun;32(6):1128-1141. doi: 10.1038/s41418-025-01462-2. Epub 2025 Feb 22. Cell Death Differ. 2025. PMID: 39987355
-
Ubiquitination of NS1 Confers Differential Adaptation of Zika Virus in Mammalian Hosts and Mosquito Vectors.Adv Sci (Weinh). 2024 Oct;11(39):e2408024. doi: 10.1002/advs.202408024. Epub 2024 Aug 19. Adv Sci (Weinh). 2024. PMID: 39159062 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

