In Silico Structure-Based Design of Antiviral Peptides Targeting the Severe Fever with Thrombocytopenia Syndrome Virus Glycoprotein Gn
- PMID: 34696477
- PMCID: PMC8539749
- DOI: 10.3390/v13102047
In Silico Structure-Based Design of Antiviral Peptides Targeting the Severe Fever with Thrombocytopenia Syndrome Virus Glycoprotein Gn
Abstract
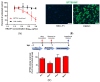
Severe fever with thrombocytopenia syndrome virus (SFTSV) is an emerging tick-borne bunyavirus in Asia that causes severe disease. Despite its clinical importance, treatment options for SFTSV infection remains limited. The SFTSV glycoprotein Gn plays a major role in mediating virus entry into host cells and is therefore a potential antiviral target. In this study, we employed an in silico structure-based strategy to design novel cyclic antiviral peptides that target the SFTSV glycoprotein Gn. Among the cyclic peptides, HKU-P1 potently neutralizes the SFTSV virion. Combinatorial treatment with HKU-P1 and the broad-spectrum viral RNA-dependent RNA polymerase inhibitor favipiravir exhibited synergistic antiviral effects in vitro. The in silico peptide design platform in this study may facilitate the generation of novel antiviral peptides for other emerging viruses.
Keywords: SFSTV; antiviral; bunyavirales; peptide; tick; treatment.
Conflict of interest statement
J.F-W.C. has received travel grants from Pfizer Corporation Hong Kong and Astellas Pharma Hong Kong Corporation Limited and was an invited speaker for Gilead Sciences Hong Kong Limited and Luminex Corporation. The other authors declared no conflict of interests. The funding sources had no role in study design, data collection, analysis or interpretation, or writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit it for publication.
Figures


References
-
- Hofmann H., Li X.X., Zhang X.A., Liu W., Kuhl A., Kaup F., Soldan S.S., Gonzalez-Scarano F., Weber F., He Y.X., et al. Severe Fever with Thrombocytopenia Virus Glycoproteins Are Targeted by Neutralizing Antibodies and Can Use DC-SIGN as a Receptor for pH-Dependent Entry into Human and Animal Cell Lines. J. Virol. 2013;87:4384–4394. doi: 10.1128/JVI.02628-12. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

