Glycan Recognition in Human Norovirus Infections
- PMID: 34696500
- PMCID: PMC8537403
- DOI: 10.3390/v13102066
Glycan Recognition in Human Norovirus Infections
Abstract
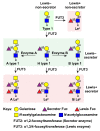
Recognition of cell-surface glycans is an important step in the attachment of several viruses to susceptible host cells. The molecular basis of glycan interactions and their functional consequences are well studied for human norovirus (HuNoV), an important gastrointestinal pathogen. Histo-blood group antigens (HBGAs), a family of fucosylated carbohydrate structures that are present on the cell surface, are utilized by HuNoVs to initially bind to cells. In this review, we describe the discovery of HBGAs as genetic susceptibility factors for HuNoV infection and review biochemical and structural studies investigating HuNoV binding to different HBGA glycans. Recently, human intestinal enteroids (HIEs) were developed as a laboratory cultivation system for HuNoV. We review how the use of this novel culture system has confirmed that fucosylated HBGAs are necessary and sufficient for infection by several HuNoV strains, describe mechanisms of antibody-mediated neutralization of infection that involve blocking of HuNoV binding to HBGAs, and discuss the potential for using the HIE model to answer unresolved questions on viral interactions with HBGAs and other glycans.
Keywords: glycoconjugates; histo-blood group antigens; host–virus interactions; human intestinal enteroids/organoids; human noroviruses; structure.
Conflict of interest statement
M.K.E. is named as an inventor on patents related to cloning of the Norwalk virus genome and HuNoV cultivation, and is a consultant to and received research funding from Takeda Vaccines, Inc. R.L.A. is named as an inventor on patents related to HuNoV cultivation and has received research funding from Takeda Vaccines, Inc. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Gagneux P., Aebi M., Varki A. Evolution of Glycan Diversity. In: Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M., Darvill A.G., Kinoshita T., Packer N.H., Prestegard J.H., et al., editors. Essentials of Glycobiology. Cold Spring Harbor Laboratory Press; New York, NY, USA: 2015–2017. pp. 253–264. [Internet] - DOI
-
- Rini J.M., Esko J.D. Glycosyltransferases and Glycan-Processing Enzymes. In: Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M., Darvill A.G., Kinoshita T., Packer N.H., Prestegard J.H., et al., editors. Essentials of Glycobiology. Cold Spring Harbor Laboratory Press; New York, NY, USA: 2015–2017. pp. 65–75. [Internet] - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

