Feasibility and Effectiveness Assessment of SARS-CoV-2 Antigenic Tests in Mass Screening of a Pediatric Population and Correlation with the Kinetics of Viral Loads
- PMID: 34696501
- PMCID: PMC8537025
- DOI: 10.3390/v13102071
Feasibility and Effectiveness Assessment of SARS-CoV-2 Antigenic Tests in Mass Screening of a Pediatric Population and Correlation with the Kinetics of Viral Loads
Abstract
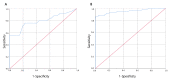
The gold standard for diagnosis of SARS-CoV-2 infection has been nucleic acid amplification tests (NAAT). However, rapid antigen detection kits (Ag-RDTs), may offer advantages over NAAT in mass screening, generating results in minutes, both as laboratory-based test or point-of-care (POC) use for clinicians, at a lower cost. We assessed two different POC Ag-RDTs in mass screening versus NAAT for SARS-CoV-2 in a cohort of pediatric patients admitted to the Pediatric Emergency Unit of IRCCS-Polyclinic of Sant'Orsola, Bologna (from November 2020 to April 2021). All patients were screened with nasopharyngeal swabs for the detection of SARS-CoV-2-RNA and for antigen tests. Results were obtained from 1146 patients. The COVID-19 Ag FIA kit showed a baseline sensitivity of 53.8% (CI 35.4-71.4%), baseline specificity 99.7% (CI 98.4-100%) and overall accuracy of 80% (95% CI 0.68-0.91); the AFIAS COVID-19 Ag kit, baseline sensitivity of 86.4% (CI 75.0-93.9%), baseline specificity 98.3% (CI 97.1-99.1%) and overall accuracy of 95.3% (95% CI 0.92-0.99). In both tests, some samples showed very low viral load and negative Ag-RDT. This disagreement may reflect the positive inability of Ag-RDTs of detecting antigen in late phase of infection. Among all cases with positive molecular test and negative antigen test, none showed viral loads > 106 copies/mL. Finally, we found one false Ag-RDTs negative result (low cycle thresholds; 9 × 105 copies/mL). Our results suggest that both Ag-RDTs showed good performances in detection of high viral load samples, making it a feasible and effective tool for mass screening in actively infected children.
Keywords: COVID-19; SARS-CoV-2; children; mass screening; rapid antigenic test; real time RT-PCR.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- European Centre for Disease Prevention and Control . COVID-19 Testing Strategies and Objectives. ECDC; Stockholm, Sweden: 2020. [(accessed on 1 July 2021)]. Available online: https://www.ecdc.europa.eu/en/publications-data/covid-19-testing-strateg....
-
- WHO Laboratory Testing for Coronavirus Disease (COVID-19) in Suspected Human Cases: Interim Guidance, 19 March 2020. [(accessed on 1 July 2021)]. Available online: https://apps.who.int/iris/handle/10665/331501.
-
- European Centre for Disease Prevention and Control/European Agency for Safety and Health at Work . Considerations on the Use of Rapid Antigen Detection (Including Self) Tests for SARS-CoV-2 in Occupational Settings. ECDC/EU-OSHA; Stockholm, Sweden: Bilbao, Spain: May 6, 2021. [(accessed on 1 July 2021)]. Available online: https://www.ecdc.europa.eu/en/publications-data/considerations-use-rapid....
-
- European Centre for Disease Prevention and Control . Options for the Use of Rapid Antigen Tests for COVID-19 in the EU/EEA and the UK. ECDC; Stockholm, Sweden: 2020. [(accessed on 1 July 2021)]. Available online: https://www.ecdc.europa.eu/en/publications-data/options-use-rapid-antige....
-
- Aggiornamento Della Definizione di Caso COVID-19 e Strategie di Testing e Screening—AGENAS. [(accessed on 1 July 2021)]; Available online: https://www.agenas.gov.it/comunicazione/primo-piano/1795-aggiornamento-d....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

