Comparison of Eight Commercially Available Faecal Point-of-Care Tests for Detection of Canine Parvovirus Antigen
- PMID: 34696513
- PMCID: PMC8540396
- DOI: 10.3390/v13102080
Comparison of Eight Commercially Available Faecal Point-of-Care Tests for Detection of Canine Parvovirus Antigen
Abstract
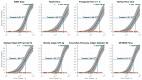
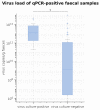
A real-time polymerase chain reaction (qPCR) is considered the gold standard for the laboratory diagnosis of canine parvovirus (CPV) infection but can only be performed in specialized laboratories. Several point-of-care tests (POCT), detecting CPV antigens in faeces within minutes, are commercially available. The aim of this study was to evaluate eight POCT in comparison with qPCR. Faecal samples of 150 dogs from three groups (H: 50 client-owned, healthy dogs, not vaccinated within the last four weeks; S: 50 shelter dogs, healthy, not vaccinated within the last four weeks; p = 50 dogs with clinical signs of CPV infection) were tested with eight POCT and qPCR. Practicability, sensitivity, specificity, positive (PPV) and negative predictive values (NPV), as well as overall accuracy were determined. To assess the differences between and agreement among POCT, McNemar's test and Cohen's Kappa statistic were performed. Specificity and PPV were 100.0% in all POCT. Sensitivity varied from 22.9-34.3% overall and from 32.7-49.0% in group P. VetexpertRapidTestCPVAg® had the highest sensitivity (34.3% overall, 49.0% group P) and differed significantly from the 3 POCT with the lowest sensitivities (Fassisi®Parvo (27.7% overall, 36.7% group P), Primagnost®ParvoH+K (24.3% overall, 34.7% group P), FASTest®PARVOCard (22.9% overall, 32.7% group P)). The agreement among all POCT was at least substantial (kappa >0.80). A positive POCT result confirmed the infection with CPV in unvaccinated dogs, whereas a negative POCT result did not definitely exclude CPV infection due to the low sensitivity of all POCT.
Keywords: CPV; POCT; diagnosis; in-house test; parvovirosis; sensitivity; specificity.
Conflict of interest statement
The authors declare no potential conflict of interest with respect to the research, authorship, and/or publication of this article. Although the POCT were provided for free by IDEXX, Fassisi, Dechra, Megacor, Bionote, Vetexpert and Zoetis, the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. There is no commercial conflict of interest as the information generated here is solely for scientific dissemination. The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

