Flavivirus Persistence in Wildlife Populations
- PMID: 34696529
- PMCID: PMC8541186
- DOI: 10.3390/v13102099
Flavivirus Persistence in Wildlife Populations
Abstract
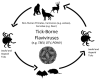
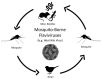
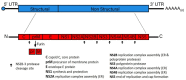
A substantial number of humans are at risk for infection by vector-borne flaviviruses, resulting in considerable morbidity and mortality worldwide. These viruses also infect wildlife at a considerable rate, persistently cycling between ticks/mosquitoes and small mammals and reptiles and non-human primates and humans. Substantially increasing evidence of viral persistence in wildlife continues to be reported. In addition to in humans, viral persistence has been shown to establish in mammalian, reptile, arachnid, and mosquito systems, as well as insect cell lines. Although a considerable amount of research has centered on the potential roles of defective virus particles, autophagy and/or apoptosis-induced evasion of the immune response, and the precise mechanism of these features in flavivirus persistence have yet to be elucidated. In this review, we present findings that aid in understanding how vector-borne flavivirus persistence is established in wildlife. Research studies to be discussed include determining the critical roles universal flavivirus non-structural proteins played in flaviviral persistence, the advancement of animal models of viral persistence, and studying host factors that allow vector-borne flavivirus replication without destructive effects on infected cells. These findings underscore the viral-host relationships in wildlife animals and could be used to elucidate the underlying mechanisms responsible for the establishment of viral persistence in these animals.
Keywords: arbovirus; autophagy; flaviviruses; infection; interferon; mosquito; tick; viral persistence; wildlife.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

