Clinical Efficacy of Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer Patients with Liver Metastases: A Network Meta-Analysis of Nine Randomized Controlled Trials
- PMID: 34696564
- PMCID: PMC9296924
- DOI: 10.4143/crt.2021.764
Clinical Efficacy of Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer Patients with Liver Metastases: A Network Meta-Analysis of Nine Randomized Controlled Trials
Abstract
Purpose: This network meta-analysis (NMA) was conducted to compare the efficacy of immune checkpoint inhibitors in advanced non-small cell lung cancer (NSCLC) patients with liver metastases.
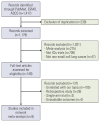
Materials and methods: English literature was retrieved from the PubMed, American Society of Clinical Oncology, and European Society for Medical Oncology databases from January 2015 to January 2021. We pooled the overall survival (OS) and progression-free survival (PFS) hazard ratios (HRs) using an NMA and ranked treatments by the surface under the cumulative ranking curve. Publication bias was evaluated by Begg's and Egger's tests. STATA 15.0 was used for the sensitivity analysis, and the remaining statistical analyses were performed using R 4.0.2.
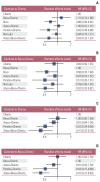
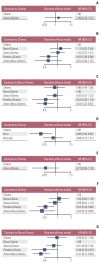
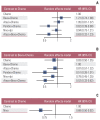
Results: Nine eligible phase III randomized controlled trials were included, including 1,141 patients with liver metastases. Pembrolizumab+chemotherapy ranked highest, followed by atezolizumab+bevacizumab+chemotherapy and nivolumab. However, no significant difference in OS rates was observed across these three treatments (HR, 0.98; 95% confidence interval [CI], 0.43 to 2.22 for pembrolizumab+chemotherapy vs. atezolizumab+bevacizumab+chemotherapy; HR, 0.91; 95% CI, 0.52 to 1.57 for pembrolizumab+chemotherapy vs. nivolumab). Regarding the PFS rate, atezolizumab+bevacizumab+chemotherapy and pembro-lizumab+chemotherapy ranked highest and no significant difference was observed between them (HR, 0.79; 95% CI, 0.36 to 1.70 for atezolizumab+bevacizumab+chemotherapy vs. pembrolizumab+chemotherapy).
Conclusion: Pembrolizumab+chemotherapy, atezolizumab+bevacizumab+chemotherapy, and nivolumab were superior to other treatments in NSCLC patients with liver metastases. These new findings may help clinicians better select therapeutic strategies for NSCLC patients with liver metastases.
Keywords: Advanced non-small cell lung cancer; Immune checkpoint inhibitors; Liver metastases; Network meta-analysis; Overall survival; Progression-free survival.
Conflict of interest statement
Conflict of interest relevant to this article was not reported.
Figures
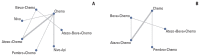
Similar articles
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2020 Dec 14;12(12):CD013257. doi: 10.1002/14651858.CD013257.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 30;4:CD013257. doi: 10.1002/14651858.CD013257.pub3. PMID: 33316104 Free PMC article. Updated.
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2021 Apr 30;4(4):CD013257. doi: 10.1002/14651858.CD013257.pub3. Cochrane Database Syst Rev. 2021. PMID: 33930176 Free PMC article.
-
Comparison of the profiles of first-line PD-1/PD-L1 inhibitors for advanced NSCLC lacking driver gene mutations: a systematic review and Bayesian network meta-analysis.Ther Adv Chronic Dis. 2023 Oct 11;14:20406223231189224. doi: 10.1177/20406223231189224. eCollection 2023. Ther Adv Chronic Dis. 2023. PMID: 37841212 Free PMC article.
-
Severe immune-related adverse events of immune checkpoint inhibitors for advanced non-small cell lung cancer: a network meta-analysis of randomized clinical trials.Cancer Immunol Immunother. 2022 Sep;71(9):2239-2254. doi: 10.1007/s00262-022-03140-5. Epub 2022 Feb 6. Cancer Immunol Immunother. 2022. PMID: 35124713 Free PMC article.
-
Selection of first-line systemic therapies for advanced hepatocellular carcinoma: A network meta-analysis of randomized controlled trials.World J Gastroenterol. 2021 May 21;27(19):2415-2433. doi: 10.3748/wjg.v27.i19.2415. World J Gastroenterol. 2021. PMID: 34040331 Free PMC article.
Cited by
-
Progress of immune checkpoint inhibitors therapy for non-small cell lung cancer with liver metastases.Br J Cancer. 2024 Feb;130(2):165-175. doi: 10.1038/s41416-023-02482-w. Epub 2023 Nov 9. Br J Cancer. 2024. PMID: 37945751 Free PMC article. Review.
-
Assessing the Relationship Between Liver Metastases and the Survival of Patients With Non-Small Cell Lung Cancer After Immune Checkpoint Inhibitors Treatment: A Systematic Review and Meta-Analysis.Integr Cancer Ther. 2023 Jan-Dec;22:15347354231164584. doi: 10.1177/15347354231164584. Integr Cancer Ther. 2023. PMID: 36998207 Free PMC article.
-
The efficacy and safety of immune combination therapy in patients with driver gene-negative non-small cell lung cancer with liver metastasis: a systematic review and network meta-analysis.BMC Cancer. 2025 Aug 18;25(1):1332. doi: 10.1186/s12885-025-14712-w. BMC Cancer. 2025. PMID: 40826400 Free PMC article.
-
Liver metastases and the efficacy of immune checkpoint inhibitors in advanced lung cancer: A systematic review and meta-analysis.Front Oncol. 2022 Oct 18;12:978069. doi: 10.3389/fonc.2022.978069. eCollection 2022. Front Oncol. 2022. PMID: 36330494 Free PMC article.
-
Effectiveness and Safety of Immune Checkpoint Inhibitors Alone or in Combination With Chemotherapy in Pulmonary Sarcomatoid Carcinoma.JTO Clin Res Rep. 2023 Nov 27;5(1):100613. doi: 10.1016/j.jtocrr.2023.100613. eCollection 2024 Jan. JTO Clin Res Rep. 2023. PMID: 38229769 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. - PubMed
-
- Shro ff GS, Viswanathan C, Carter BW, Benveniste MF, Truong MT, Sabloff BS. Staging lung cancer: metastasis. Radiol Clin North Am. 2018;56:411–8. - PubMed
-
- Morgensztern D, Waqar S, Subramanian J, Gao F, Govindan R. Improving survival for stage IV non-small cell lung cancer: a surveillance, epidemiology, and end results survey from 1990 to 2005. J Thorac Oncol. 2009;4:1524–9. - PubMed
-
- Morgensztern D, Ng SH, Gao F, Govindan R. Trends in stage distribution for patients with non-small cell lung cancer: a National Cancer Database survey. J Thorac Oncol. 2010;5:29–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

