Clinical Efficacy of Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer Patients with Liver Metastases: A Network Meta-Analysis of Nine Randomized Controlled Trials
- PMID: 34696564
- PMCID: PMC9296924
- DOI: 10.4143/crt.2021.764
Clinical Efficacy of Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer Patients with Liver Metastases: A Network Meta-Analysis of Nine Randomized Controlled Trials
Abstract
Purpose: This network meta-analysis (NMA) was conducted to compare the efficacy of immune checkpoint inhibitors in advanced non-small cell lung cancer (NSCLC) patients with liver metastases.
Materials and methods: English literature was retrieved from the PubMed, American Society of Clinical Oncology, and European Society for Medical Oncology databases from January 2015 to January 2021. We pooled the overall survival (OS) and progression-free survival (PFS) hazard ratios (HRs) using an NMA and ranked treatments by the surface under the cumulative ranking curve. Publication bias was evaluated by Begg's and Egger's tests. STATA 15.0 was used for the sensitivity analysis, and the remaining statistical analyses were performed using R 4.0.2.
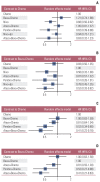
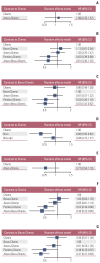
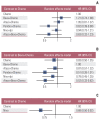
Results: Nine eligible phase III randomized controlled trials were included, including 1,141 patients with liver metastases. Pembrolizumab+chemotherapy ranked highest, followed by atezolizumab+bevacizumab+chemotherapy and nivolumab. However, no significant difference in OS rates was observed across these three treatments (HR, 0.98; 95% confidence interval [CI], 0.43 to 2.22 for pembrolizumab+chemotherapy vs. atezolizumab+bevacizumab+chemotherapy; HR, 0.91; 95% CI, 0.52 to 1.57 for pembrolizumab+chemotherapy vs. nivolumab). Regarding the PFS rate, atezolizumab+bevacizumab+chemotherapy and pembro-lizumab+chemotherapy ranked highest and no significant difference was observed between them (HR, 0.79; 95% CI, 0.36 to 1.70 for atezolizumab+bevacizumab+chemotherapy vs. pembrolizumab+chemotherapy).
Conclusion: Pembrolizumab+chemotherapy, atezolizumab+bevacizumab+chemotherapy, and nivolumab were superior to other treatments in NSCLC patients with liver metastases. These new findings may help clinicians better select therapeutic strategies for NSCLC patients with liver metastases.
Keywords: Advanced non-small cell lung cancer; Immune checkpoint inhibitors; Liver metastases; Network meta-analysis; Overall survival; Progression-free survival.
Conflict of interest statement
Conflict of interest relevant to this article was not reported.
Figures
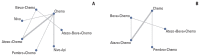
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. - PubMed
-
- Shro ff GS, Viswanathan C, Carter BW, Benveniste MF, Truong MT, Sabloff BS. Staging lung cancer: metastasis. Radiol Clin North Am. 2018;56:411–8. - PubMed
-
- Morgensztern D, Waqar S, Subramanian J, Gao F, Govindan R. Improving survival for stage IV non-small cell lung cancer: a surveillance, epidemiology, and end results survey from 1990 to 2005. J Thorac Oncol. 2009;4:1524–9. - PubMed
-
- Morgensztern D, Ng SH, Gao F, Govindan R. Trends in stage distribution for patients with non-small cell lung cancer: a National Cancer Database survey. J Thorac Oncol. 2010;5:29–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

