Quality of biosafety guidelines for dental clinical practice throughout the world in the early COVID-19 pandemic: a systematic review
- PMID: 34696570
- PMCID: PMC8920742
- DOI: 10.4178/epih.e2021089
Quality of biosafety guidelines for dental clinical practice throughout the world in the early COVID-19 pandemic: a systematic review
Abstract
Objectives: To conduct a systematic review of coronavirus disease 2019 (COVID-19)-related biosafety guidelines for dental clinical practice in the early stage of the pandemic, focusing on quality assessment.
Methods: Electronic (via PubMed, Scopus, Web of Science, Latin American and Caribbean Health Sciences Literature database, Brazilian Library in Dentistry, and Cochrane Library) and gray literature searches were performed for documents published up to May 12, 2020. Guidelines updated until April 17, 2021 were identified. Documents were included as guidelines if they (1) consisted of a set of statements, directions, or principles presenting current or future rules or policy; (2) were developed by government agencies, institutions, organizations, or expert panels; and (3) were related to the general conduct of healthcare activities rather a particular condition. Two researchers, using the Appraisal of Guidelines for Research & Evaluation II, independently extracted the recommendations and evaluated the quality of the guidelines.
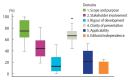
Results: Twenty-seven documents from 19 countries were included in the review. These documents presented 122 recommendations related to (1) professional biosafety; (2) patients'/companions' safety; (3) the organization and biosafety of the physical dental facility environment; and (4) the work process in dental care. Overall, the scientific quality of the guidelines was considered low. Some recommendations presented in these guidelines would require further research to establish their effectiveness.
Conclusions: We found a wide variety of biosafety guidelines for dental practice regarding COVID-19 in the early months of the pandemic, but their quality was low. Biosafety recommendations should be frequently updated.
Keywords: Biosafety; COVID-19; Dentistry; Guidelines; SARS-CoV-2.
Conflict of interest statement
The authors have no conflicts of interest to declare for this study.
Figures

References
-
- Mahase E. China coronavirus: WHO declares international emergency as death toll exceeds 200. BMJ. 2020;368:m408. - PubMed
-
- World Health Organization Virtual press conference on COVID-19. 2020 Mar 11 [cited 2021 Mar 20]. Available from: https://www.who.int/docs/default-source/coronaviruse/transcripts/who-aud....
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

