Pathogenesis and virulence of flavivirus infections
- PMID: 34696709
- PMCID: PMC8632085
- DOI: 10.1080/21505594.2021.1996059
Pathogenesis and virulence of flavivirus infections
Abstract
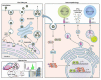
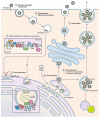
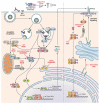
The Flavivirus genus consists of >70 members including several that are considered significant human pathogens. Flaviviruses display a broad spectrum of diseases that can be roughly categorised into two phenotypes - systemic disease involving haemorrhage exemplified by dengue and yellow Fever virus, and neurological complications associated with the likes of West Nile and Zika viruses. Attempts to develop vaccines have been variably successful against some. Besides, mosquito-borne flaviviruses can be vertically transmitted in the arthropods, enabling long term persistence and the possibility of re-emergence. Therefore, developing strategies to combat disease is imperative even if vaccines become available. The cellular interactions of flaviviruses with their human hosts are key to establishing the viral lifecycle on the one hand, and activation of host immunity on the other. The latter should ideally eradicate infection, but often leads to immunopathological and neurological consequences. In this review, we use Dengue and Zika viruses to discuss what we have learned about the cellular and molecular determinants of the viral lifecycle and the accompanying immunopathology, while highlighting current knowledge gaps which need to be addressed in future studies.
Keywords: Dengue; Flavivirus; Zika; host-pathogen interactions; immunity; life-cycle; pathogenesis.
Conflict of interest statement
No potential conflict of interest was reported by the author
Figures

References
-
- Gould E, Solomon T.. Pathogenic flaviviruses. Lancet. 2008;371:500–509. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
