Dynamic Rhythmogenic Network States Drive Differential Opioid Responses in the In Vitro Respiratory Network
- PMID: 34697095
- PMCID: PMC8638687
- DOI: 10.1523/JNEUROSCI.1329-21.2021
Dynamic Rhythmogenic Network States Drive Differential Opioid Responses in the In Vitro Respiratory Network
Abstract
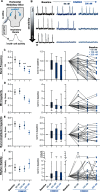
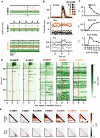
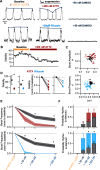
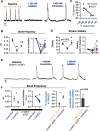
Death from opioid overdose is typically caused by opioid-induced respiratory depression (OIRD). A particularly dangerous characteristic of OIRD is its apparent unpredictability. The respiratory consequences of opioids can be surprisingly inconsistent, even within the same individual. Despite significant clinical implications, most studies have focused on average dose-r esponses rather than individual variation, and there remains little insight into the etiology of this apparent unpredictability. The preBötzinger complex (preBötC) in the ventral medulla is an important site for generating the respiratory rhythm and OIRD. Here, using male and female C57-Bl6 mice in vitro, we demonstrate that the preBötC can assume different network states depending on the excitability of the preBötC and the intrinsic membrane properties of preBötC neurons. These network states predict the functional consequences of opioids in the preBötC, and depending on network state, respiratory rhythmogenesis can be either stabilized or suppressed by opioids. We hypothesize that the dynamic nature of preBötC rhythmogenic properties, required to endow breathing with remarkable flexibility, also plays a key role in the dangerous unpredictability of OIRD.SIGNIFICANCE STATEMENT Opioids can cause unpredictable, life-threatening suppression of breathing. This apparent unpredictability makes clinical management of opioids difficult while also making it challenging to define the underlying mechanisms of OIRD. Here, we find in brainstem slices that the preBötC, an opioid-sensitive subregion of the brainstem, has an optimal configuration of cellular and network properties that results in a maximally stable breathing rhythm. These properties are dynamic, and the state of each individual preBötC network relative to the optimal configuration of the network predicts how vulnerable rhythmogenesis is to the effects of opioids. These insights establish a framework for understanding how endogenous and exogenous modulation of the rhythmogenic state of the preBötC can increase or decrease the risk of OIRD.
Keywords: network; opioid; preBötzinger; respiratory; rhythm; stability.
Copyright © 2021 the authors.
Figures


Similar articles
-
Modeling Effects of Variable preBötzinger Complex Network Topology and Cellular Properties on Opioid-Induced Respiratory Depression and Recovery.eNeuro. 2024 Mar 7;11(3):ENEURO.0284-23.2023. doi: 10.1523/ENEURO.0284-23.2023. Print 2024 Mar. eNeuro. 2024. PMID: 38253582 Free PMC article.
-
Opioids modulate an emergent rhythmogenic process to depress breathing.Elife. 2019 Dec 16;8:e50613. doi: 10.7554/eLife.50613. Elife. 2019. PMID: 31841107 Free PMC article.
-
Dual mechanisms of opioid-induced respiratory depression in the inspiratory rhythm-generating network.Elife. 2021 Aug 17;10:e67523. doi: 10.7554/eLife.67523. Elife. 2021. PMID: 34402425 Free PMC article.
-
Mechanisms of opioid-induced respiratory depression.Arch Toxicol. 2022 Aug;96(8):2247-2260. doi: 10.1007/s00204-022-03300-7. Epub 2022 Apr 26. Arch Toxicol. 2022. PMID: 35471232 Review.
-
Opioid-induced respiratory depression: clinical aspects and pathophysiology of the respiratory network effects.Am J Physiol Lung Cell Mol Physiol. 2025 Feb 1;328(2):L267-L289. doi: 10.1152/ajplung.00314.2024. Epub 2024 Dec 27. Am J Physiol Lung Cell Mol Physiol. 2025. PMID: 39726397 Review.
Cited by
-
Breathing Rhythm and Pattern and Their Influence on Emotion.Annu Rev Neurosci. 2022 Jul 8;45:223-247. doi: 10.1146/annurev-neuro-090121-014424. Epub 2022 Mar 8. Annu Rev Neurosci. 2022. PMID: 35259917 Free PMC article. Review.
-
Modeling Effects of Variable preBötzinger Complex Network Topology and Cellular Properties on Opioid-Induced Respiratory Depression and Recovery.eNeuro. 2024 Mar 7;11(3):ENEURO.0284-23.2023. doi: 10.1523/ENEURO.0284-23.2023. Print 2024 Mar. eNeuro. 2024. PMID: 38253582 Free PMC article.
-
Inspiratory rhythm generation is stabilized by Ih.J Neurophysiol. 2022 Jul 1;128(1):181-196. doi: 10.1152/jn.00150.2022. Epub 2022 Jun 8. J Neurophysiol. 2022. PMID: 35675444 Free PMC article.
-
Purinergic signaling mediates neuroglial interactions to modulate sighs.Nat Commun. 2023 Aug 31;14(1):5300. doi: 10.1038/s41467-023-40812-x. Nat Commun. 2023. PMID: 37652903 Free PMC article.
-
Disinhibition does not play a role in endomorphin-2-induced changes in inspiratory motoneuron output produced by in vitro neonatal rat preparations.Respir Physiol Neurobiol. 2024 Feb;320:104186. doi: 10.1016/j.resp.2023.104186. Epub 2023 Nov 7. Respir Physiol Neurobiol. 2024. PMID: 37944625 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
