Immune landscape in vulvar cancer-draining lymph nodes indicates distinct immune escape mechanisms in support of metastatic spread and growth
- PMID: 34697217
- PMCID: PMC8547515
- DOI: 10.1136/jitc-2021-003623
Immune landscape in vulvar cancer-draining lymph nodes indicates distinct immune escape mechanisms in support of metastatic spread and growth
Abstract
Background: Therapeutic immune intervention is highly dependent on the T-cell priming and boosting capacity of tumor-draining lymph nodes (TDLN). In vulvar cancer, in-depth studies on the immune status of (pre)metastatic TDLN is lacking.
Methods: We have phenotyped and enumerated various T-cell and myeloid subsets in tumor-free (LN-, n=27) and metastatic TDLN (LN+, n=11) using flow cytometry. Additionally, we studied chemokine and cytokine release profiles and assessed expression of indoleamine 2,3-dioxygenase (IDO) in relation to plasmacytoid dendritic cell (pDC) or myeloid subsets.
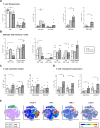
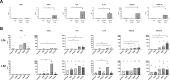
Results: Metastatic involvement of TDLN was accompanied by an inflamed microenvironment with immune suppressive features, marked by hampered activation of migratory DC, increased cytokine/chemokine release, and closely correlated elevations of pDC and LN-resident conventional DC (LNR-cDC) activation state and frequencies, as well as of terminal CD8+ effector-memory T-cell (TemRA) differentiation, regulatory T-cell (Treg) rates, T-cell activation, and expression of cytotoxic T-lymphocyte protein-4 (CTLA-4) and programmed cell death protein-1 (PD-1) immune checkpoints. In addition, high indoleamine 2,3-dioxygenase (IDO) expression and increased frequencies of monocytic myeloid-derived suppressor cells (mMDSC) were observed. Correlation analyses with primary and metastatic tumor burden suggested respective roles for Tregs and suppression of inducible T cell costimulator (ICOS)+ T helper cells in early metastatic niche formation and for CD14+ LNR-cDC and terminal T-cell differentiation in later stages of metastatic growth.
Conclusions: Metastatic spread in vulvar TDLN is marked by an inflamed microenvironment with activated effector T cells, which are likely kept in check by an interplay of suppressive feedback mechanisms. Our data support (neoadjuvant) TDLN-targeted therapeutic interventions based on CTLA-4 and PD-1 blockade, to reinvigorate memory T cells and curb early metastatic spread and growth.
Keywords: T-lymphocytes; cytokines; dendritic cells; myeloid-derived suppressor cells; tumor microenvironment.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
