Effects of exercise on AKT/PGC1-α/FOXO3a pathway and muscle atrophy in cisplatin-administered rat skeletal muscle
- PMID: 34697269
- PMCID: PMC8552830
- DOI: 10.4196/kjpp.2021.25.6.585
Effects of exercise on AKT/PGC1-α/FOXO3a pathway and muscle atrophy in cisplatin-administered rat skeletal muscle
Abstract
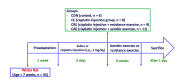
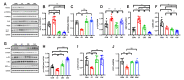
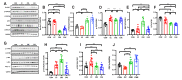
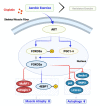
Cisplatin has been reported to cause side effects such as muscle wasting in humans and rodents. The physiological mechanisms involved in preventing muscle wasting, such as the regulation of AKT, PGC1-α, and autophagy-related factor FOXO3a by MuRF 1 and Atrogin-1, remain unclear following different types of exercise and in various skeletal muscle types. Eight-week-old male Wistar rats (n = 34) were assigned to one of four groups: control (CON, n = 6), cisplatin injection (1 mg/kg) without exercise (CC, n = 8), cisplatin (1 mg/kg) + resistance exercise (CRE, n = 9) group, and cisplatin (1 mg/kg) + aerobic exercise (CAE, n = 11). The CRE group performed progressive ladder exercise (starting with 10% of body weight on a 1-m ladder with 2-cm-interval grids, at 85°) for 8 weeks. The CAE group exercised by treadmill running (20 m/min for 60 min daily, 4 times/week) for 8 weeks. Compared with the CC group, the levels of the autophagy-related factors BNIP3, Beclin 1, LC3-II/I ratio, p62, and FOXO3a in the gastrocnemius and soleus muscles were significantly decreased in the CRE and CAE groups. The CRE and CAE groups further showed significantly decreased MuRF 1 and Atrogin-1 levels and increased phosphorylation of AKT, FOXO3a, and PGC1-α. These results suggest that both ladder and aerobic exercise directly affected muscle wasting by modulating the AKT/PGC1-α/FOXO3a signaling pathways regardless of the skeletal muscle type.
Keywords: Autophagy; Cisplatin; Exercise training; Muscle atrophy; Skeletal muscle.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. - DOI - PubMed
-
- Galluzzi L, Baehrecke EH, Ballabio A, Boya P, Bravo-San Pedro JM, Cecconi F, Choi AM, Chu CT, Codogno P, Colombo MI, Cuervo AM, Debnath J, Deretic V, Dikic I, Eskelinen EL, Fimia GM, Fulda S, Gewirtz DA, Green DR, Hansen M, et al. Molecular definitions of autophagy and related processes. EMBO J. 2017;36:1811–1836. doi: 10.15252/embj.201796697. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

