Influences of ethanol and temperature on sucrose-evoked response of gustatory neurons in the hamster solitary nucleus
- PMID: 34697271
- PMCID: PMC8552825
- DOI: 10.4196/kjpp.2021.25.6.603
Influences of ethanol and temperature on sucrose-evoked response of gustatory neurons in the hamster solitary nucleus
Abstract
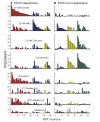
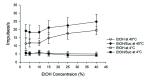
Taste-responsive neurons in the nucleus of the solitary tract (NST), the first gustatory nucleus, often respond to thermal or mechanical stimulation. Alcohol, not a typical taste modality, is a rewarding stimulus. In this study, we aimed to investigate the effects of ethanol (EtOH) and/or temperature as stimuli to the tongue on the activity of taste-responsive neurons in hamster NST. In the first set of experiments, we recorded the activity of 113 gustatory NST neurons in urethane-anesthetized hamsters and evaluated responses to four basic taste stimuli, 25% EtOH, and 40°C and 4°C distilled water (dH2O). Sixty cells responded to 25% EtOH, with most of them also being sucrose sensitive. The response to 25% EtOH was significantly correlated with the sucrose-evoked response. A significant correlation was also observed between sucrose- and 40°C dH2O-and between 25% EtOH- and 40°C dH2O-evoked firings. In a subset of the cells, we evaluated neuronal activities in response to a series of EtOH concentrations, alone and in combination with 32 mM sucrose (EtOH/Suc) at room temperature (RT, 22°C-23°C), 40°C, and 4°C. Neuronal responses to EtOH at RT and 40°C increased as the concentrations increased. The firing rates to EtOH/Suc were greater than those to EtOH or sucrose alone. The responses were enhanced when solutions were applied at 40°C but diminished at 4°C. In summary, EtOH activates most sucrose-responsive NST gustatory cells, and the concomitant presence of sucrose or warm temperatures enhance this response. Our findings may contribute to elucidate the neural mechanisms underlying appetitive alcohol consumption.
Keywords: Electrophysiology; Ethanol; In vivo; Solitary nucleus; Sucrose.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Lundy RF., Jr . Gustatory system. In: Paxinos G, editor. The rat nervous system. 3rd ed. Elsevier Academic Press; Cambridge: 2004. pp. 891–921. - DOI
-
- Rolls ET. The brain and emotion. Oxford University Press; New York: 1999.

