Pparg signaling controls bladder cancer subtype and immune exclusion
- PMID: 34697317
- PMCID: PMC8545976
- DOI: 10.1038/s41467-021-26421-6
Pparg signaling controls bladder cancer subtype and immune exclusion
Abstract
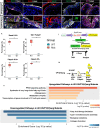
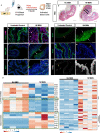
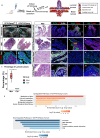
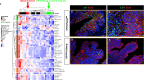
Pparg, a nuclear receptor, is downregulated in basal subtype bladder cancers that tend to be muscle invasive and amplified in luminal subtype bladder cancers that tend to be non-muscle invasive. Bladder cancers derive from the urothelium, one of the most quiescent epithelia in the body, which is composed of basal, intermediate, and superficial cells. We find that expression of an activated form of Pparg (VP16;Pparg) in basal progenitors induces formation of superficial cells in situ, that exit the cell cycle, and do not form tumors. Expression in basal progenitors that have been activated by mild injury however, results in luminal tumor formation. We find that these tumors are immune deserted, which may be linked to down-regulation of Nf-kb, a Pparg target. Interestingly, some luminal tumors begin to shift to basal subtype tumors with time, down-regulating Pparg and other luminal markers. Our findings have important implications for treatment and diagnosis of bladder cancer.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Society AC. Cancer Facts & Figures 2020. American Cancer Society (2020).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

