Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection
- PMID: 34697502
- PMCID: PMC8629105
- DOI: 10.1038/s41591-021-01556-7
Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection
Erratum in
-
Publisher Correction: Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection.Nat Med. 2021 Dec;27(12):2249. doi: 10.1038/s41591-021-01644-8. Nat Med. 2021. PMID: 34845390 Free PMC article. No abstract available.
Abstract
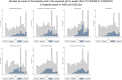
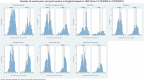
Emerging reports of rare neurological complications associated with COVID-19 infection and vaccinations are leading to regulatory, clinical and public health concerns. We undertook a self-controlled case series study to investigate hospital admissions from neurological complications in the 28 days after a first dose of ChAdOx1nCoV-19 (n = 20,417,752) or BNT162b2 (n = 12,134,782), and after a SARS-CoV-2-positive test (n = 2,005,280). There was an increased risk of Guillain-Barré syndrome (incidence rate ratio (IRR), 2.90; 95% confidence interval (CI): 2.15-3.92 at 15-21 days after vaccination) and Bell's palsy (IRR, 1.29; 95% CI: 1.08-1.56 at 15-21 days) with ChAdOx1nCoV-19. There was an increased risk of hemorrhagic stroke (IRR, 1.38; 95% CI: 1.12-1.71 at 15-21 days) with BNT162b2. An independent Scottish cohort provided further support for the association between ChAdOx1nCoV and Guillain-Barré syndrome (IRR, 2.32; 95% CI: 1.08-5.02 at 1-28 days). There was a substantially higher risk of all neurological outcomes in the 28 days after a positive SARS-CoV-2 test including Guillain-Barré syndrome (IRR, 5.25; 95% CI: 3.00-9.18). Overall, we estimated 38 excess cases of Guillain-Barré syndrome per 10 million people receiving ChAdOx1nCoV-19 and 145 excess cases per 10 million people after a positive SARS-CoV-2 test. In summary, although we find an increased risk of neurological complications in those who received COVID-19 vaccines, the risk of these complications is greater following a positive SARS-CoV-2 test.
© 2021. The Author(s).
Conflict of interest statement
A.S. is a member of the Scottish Government Chief Medical Officer’s COVID-19 Advisory Group, the Scottish Government’s Standing Committee on Pandemics, and AstraZeneca’s Thrombotic Thrombocytopenic Advisory Group. All roles are unremunerated. D.H. is a member of the Commission on Human Medicine’s Neurology, Pain and Psychiatry Expert Advisory Group. K.K. is a member of the Government Scientific Advisory Group for Emergencies. C.R. is a member of the Scottish Government Chief Medical Officer’s COVID-19 Advisory Group, SPI-M, and the MHRA Vaccine Benefit and Risk Committee. J.H.-C. reports grants from the National Institute for Health Research (NIHR) Biomedical Research Centre, Oxford, grants from John Fell Oxford University Press Research Fund, grants from Cancer Research UK (CR-UK) (grant number C5255/A18085), through the Cancer Research UK Oxford Centre, grants from the Oxford Wellcome Institutional Strategic Support Fund (204826/Z/16/Z) and other research councils, during the conduct of the study. J.H.-C. is an unpaid director of QResearch, a not-for-profit organization that is a partnership between the University of Oxford and EMIS Health, who supply the QResearch database used for this work. J.H.-C. is a founder and shareholder of ClinRisk Ltd and was its medical director until 31 May 2019. ClinRisk Ltd produces open and closed source software to implement clinical risk algorithms (outside this work) into clinical computer systems. J.H.-C. is chair of the NERVTAG risk stratification subgroup and a member of SAGE COVID-19 groups and the NHS group advising on prioritization of use of monoclonal antibodies in COVID-19 infection. All other authors have no competing interests.
Figures





Comment in
-
Comparing neurological complications after COVID-19 vaccination and SARS-CoV-2 infection.Nat Rev Immunol. 2021 Dec;21(12):761. doi: 10.1038/s41577-021-00655-3. Nat Rev Immunol. 2021. PMID: 34741166 Free PMC article. No abstract available.
-
Risk-benefit analysis of COVID-19 vaccines - a neurological perspective.Nat Rev Neurol. 2022 Feb;18(2):69-70. doi: 10.1038/s41582-021-00606-5. Nat Rev Neurol. 2022. PMID: 34931027 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

