GlcNAc-Asn is a biomarker for NGLY1 deficiency
- PMID: 34697629
- PMCID: PMC8863169
- DOI: 10.1093/jb/mvab111
GlcNAc-Asn is a biomarker for NGLY1 deficiency
Erratum in
-
Erratum.J Biochem. 2022 Mar 31;171(4):469. doi: 10.1093/jb/mvac016. J Biochem. 2022. PMID: 35181785 Free PMC article. No abstract available.
Abstract
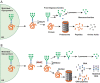
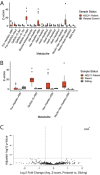
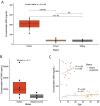
Substrate-derived biomarkers are necessary in slowly progressing monogenetic diseases caused by single-enzyme deficiencies to identify affected patients and serve as surrogate markers for therapy response. N-glycanase 1 (NGLY1) deficiency is an ultra-rare autosomal recessive disorder characterized by developmental delay, peripheral neuropathy, elevated liver transaminases, hyperkinetic movement disorder and (hypo)-alacrima. We demonstrate that N-acetylglucosamine-asparagine (GlcNAc-Asn; GNA), is the analyte most closely associated with NGLY1 deficiency, showing consistent separation in levels between patients and controls. GNA accumulation is directly linked to the absence of functional NGLY1, presenting strong potential for its use as a biomarker. In agreement, a quantitative liquid chromatography with tandem mass spectrometry assay, developed to assess GNA from 3 to 3000 ng/ml, showed that it is conserved as a marker for loss of NGLY1 function in NGLY1-deficient cell lines, rodents (urine, cerebrospinal fluid, plasma and tissues) and patients (plasma and urine). Elevated GNA levels differentiate patients from controls, are stable over time and correlate with changes in NGLY1 activity. GNA as a biomarker has the potential to identify and validate patients with NGLY1 deficiency, act as a direct pharmacodynamic marker and serve as a potential surrogate endpoint in clinical trials.
Keywords: GNA; GlcNAc-Asn; N-glycanase 1; NGLY1 deficiency; biomarker.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Japanese Biochemical Society.
Figures



References
-
- NORD . (2021) NGLY1 Deficiency, https://rarediseases.org/rare-diseases/ngly1-deficiency/, (May 24, 2021, date last accessed)
-
- Enns, G.M., Shashi, V., Bainbridge, M., Gambello, M.J., Zahir, F.R., Bast, T., Crimian, R., Schoch, K., Platt, J., Cox, R., Bernstein, J.A., Scavina, M., Walter, R.S., Bibb, A., Jones, M., Hegde, M., Graham, B.H., Need, A.C., Oviedo, A., Schaaf, C.P., Boyle, S., Butte, A.J., Chen, R., Chen, R., Clark, M.J., Haraksingh, R., Consortium, F.C., Cowan, T.M., He, P., Langlois, S., Zoghbi, H.Y., Snyder, M., Gibbs, R.A., Freeze, H.H., and Goldstein, D.B. (2014) Mutations in NGLY1 cause an inherited disorder of the endoplasmic reticulum-associated degradation pathway. Genet. Med. 16, 751–758 - PMC - PubMed
-
- Adams, J., and Schaaf, C.P. (2018) Diagnosis and genetics of alacrima. Clin. Genet. 94, 54–60 - PubMed

