Interlaboratory assessment of quantification of SARS-CoV-2 RNA by reverse transcription digital PCR
- PMID: 34697653
- PMCID: PMC8545465
- DOI: 10.1007/s00216-021-03680-2
Interlaboratory assessment of quantification of SARS-CoV-2 RNA by reverse transcription digital PCR
Abstract
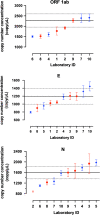
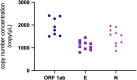
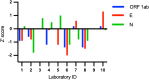
The pandemic of the novel coronavirus disease 2019 (COVID-19) has caused severe harm to the health of people all around the world. Molecular detection of the pathogen, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), played a crucial role in the control of the disease. Reverse transcription digital PCR (RT-dPCR) has been developed and used in the detection of SARS-CoV-2 RNA as an absolute quantification method. Here, an interlaboratory assessment of quantification of SARS-CoV-2 RNA was organized by the National Institute of Metrology, China (NIMC), using in vitro transcribed RNA samples, among ten laboratories on six different dPCR platforms. Copy number concentrations of three genes of SARS-CoV-2 were measured by all participants. Consistent results were obtained with dispersion within 2.2-fold and CV% below 23% among different dPCR platforms and laboratories, and Z' scores of all the reported results being satisfactory. Possible reasons for the dispersion included PCR assays, partition volume, and reverse transcription conditions. This study demonstrated the comparability and applicability of RT-dPCR method for quantification of SARS-CoV-2 RNA and showed the capability of the participating laboratories at SARS-CoV-2 test by RT-dPCR platform.
Keywords: Copy number; Quantification; RT-dPCR; SARS-CoV-2.
© 2021. Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Accurate quantification of SARS-CoV-2 RNA by isotope dilution mass spectrometry and providing a correction of reverse transcription efficiency in droplet digital PCR.Anal Bioanal Chem. 2022 Sep;414(23):6771-6777. doi: 10.1007/s00216-022-04238-6. Epub 2022 Aug 9. Anal Bioanal Chem. 2022. PMID: 35941317 Free PMC article.
-
Digital PCR Applications in the SARS-CoV-2/COVID-19 Era: a Roadmap for Future Outbreaks.Clin Microbiol Rev. 2022 Sep 21;35(3):e0016821. doi: 10.1128/cmr.00168-21. Epub 2022 Mar 8. Clin Microbiol Rev. 2022. PMID: 35258315 Free PMC article. Review.
-
Highly accurate and sensitive diagnostic detection of SARS-CoV-2 by digital PCR.Talanta. 2021 Mar 1;224:121726. doi: 10.1016/j.talanta.2020.121726. Epub 2020 Oct 27. Talanta. 2021. PMID: 33379001 Free PMC article.
-
Correlating qRT-PCR, dPCR and Viral Titration for the Identification and Quantification of SARS-CoV-2: A New Approach for Infection Management.Viruses. 2021 May 28;13(6):1022. doi: 10.3390/v13061022. Viruses. 2021. PMID: 34071726 Free PMC article.
-
Differentials of SARS-CoV-2 Viral RNA Re-positivity in Discharged COVID-19 Patients.AIDS Rev. 2021 Jun 3;23(3):153-163. doi: 10.24875/AIDSRev.21000023. AIDS Rev. 2021. PMID: 34082440 Review.
Cited by
-
A culture-free method for rapidly and accurately quantifying active SARS-CoV-2.Anal Bioanal Chem. 2023 Sep;415(23):5745-5753. doi: 10.1007/s00216-023-04855-9. Epub 2023 Jul 24. Anal Bioanal Chem. 2023. PMID: 37486370
-
Accurate quantification of SARS-CoV-2 RNA by isotope dilution mass spectrometry and providing a correction of reverse transcription efficiency in droplet digital PCR.Anal Bioanal Chem. 2022 Sep;414(23):6771-6777. doi: 10.1007/s00216-022-04238-6. Epub 2022 Aug 9. Anal Bioanal Chem. 2022. PMID: 35941317 Free PMC article.
-
Development of highly accurate digital PCR method and reference material for monkeypox virus detection.Anal Bioanal Chem. 2023 Mar;415(7):1333-1337. doi: 10.1007/s00216-023-04518-9. Epub 2023 Jan 21. Anal Bioanal Chem. 2023. PMID: 36680591 Free PMC article.
-
Digital PCR: from early developments to its future application in clinics.Lab Chip. 2025 Aug 5;25(16):3921-3961. doi: 10.1039/d5lc00055f. Lab Chip. 2025. PMID: 40686367 Free PMC article. Review.
-
Digital PCR Applications in the SARS-CoV-2/COVID-19 Era: a Roadmap for Future Outbreaks.Clin Microbiol Rev. 2022 Sep 21;35(3):e0016821. doi: 10.1128/cmr.00168-21. Epub 2022 Mar 8. Clin Microbiol Rev. 2022. PMID: 35258315 Free PMC article. Review.
References
-
- WHO. WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/. 2021.08.18.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

