A High-Throughput Chemical Screen in DJ-1β Mutant Flies Identifies Zaprinast as a Potential Parkinson's Disease Treatment
- PMID: 34697772
- PMCID: PMC8804136
- DOI: 10.1007/s13311-021-01134-2
A High-Throughput Chemical Screen in DJ-1β Mutant Flies Identifies Zaprinast as a Potential Parkinson's Disease Treatment
Abstract
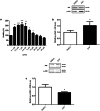
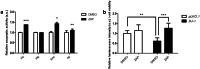
Dopamine replacement represents the standard therapy for Parkinson's disease (PD), a common, chronic, and incurable neurological disorder; however, this approach only treats the symptoms of this devastating disease. In the search for novel disease-modifying therapies that target other relevant molecular and cellular mechanisms, Drosophila has emerged as a valuable tool to study neurodegenerative diseases due to the presence of a complex central nervous system, the blood-brain barrier, and a similar neurotransmitter profile to humans. Human PD-related genes also display conservation in flies; DJ-1β is the fly ortholog of DJ-1, a gene for which mutations prompt early-onset recessive PD. Interestingly, flies mutant for DJ-1β exhibit PD-related phenotypes, including motor defects, high oxidative stress (OS) levels and metabolic alterations. To identify novel therapies for PD, we performed an in vivo high-throughput screening assay using DJ-1β mutant flies and compounds from the Prestwick® chemical library. Drugs that improved motor performance in DJ-1ß mutant flies were validated in DJ-1-deficient human neural-like cells, revealing that zaprinast displayed the most significant ability to suppress OS-induced cell death. Zaprinast inhibits phosphodiesterases and activates GPR35, an orphan G-protein-coupled receptor not previously associated with PD. We found that zaprinast exerts its beneficial effect in both fly and human PD models through several disease-modifying mechanisms, including reduced OS levels, attenuated apoptosis, increased mitochondrial viability, and enhanced glycolysis. Therefore, our results support zaprinast as a potential therapeutic for PD in future clinical trials.
Keywords: Drosophila; GPR35 agonist; High-throughput screening; Parkinson’s disease; Phosphodiesterase inhibitor; Zaprinast.
© 2021. The Author(s).
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

